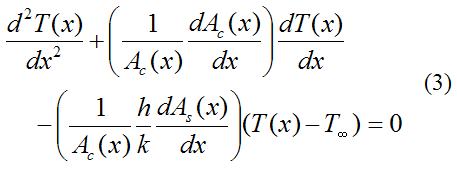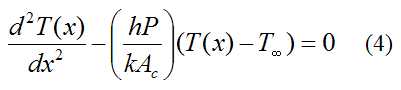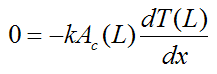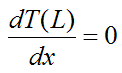Journal of Toxicology
Volume 2011 (2011), Article ID 487074, 9 pages
doi:10.1155/2011/487074Review ArticleOxidative Stress and Air Pollution ExposureMaura Lodovici and Elisabetta BigagliDepartment of Pharmacology and Toxicology, University of Florence, Viale Pieraccini 6, 50139 Florence, ItalyReceived 15 December 2010; Revised 10 May 2011; Accepted 30 June 2011Academic Editor: Susan Sumner Copyright © 2011 Maura Lodovici and Elisabetta Bigagli. .AbstractAir pollution is associated with increased cardiovascular and pulmonary morbidity and mortality.
The mechanisms of air pollution-induced health effects involve oxidative stress and inflammation. As a matter of fact, particulate matter (PM), especially fine (PM2.5, PM < 2.5 μm) and ultrafine (PM0.1, PM < 0.1 μm) particles, ozone, nitrogen oxides, and transition metals, are potent oxidants or able to generate reactive oxygen species (ROS). Oxidative stress can trigger redox-sensitive pathways that lead to different biological processes such as inflammation and cell death. However, it does appear that the susceptibility of target organ to oxidative injury also depends upon its ability to upregulate protective scavenging systems. As vehicular traffic is known to importantly contribute to PM exposure, its intensity and quality must be strongly relevant determinants of the qualitative characteristics of PM spread in the atmosphere. Change in the composition of this PM is likely to modify its health impact.1. IntroductionNumerous epidemiological studies have shown an increased morbidity and mortality due to environmental air pollution [1, 2]. Environmental air does contain a complex mixture of toxics, including particulate matter (PM), irritant gases, and benzene. The chemical composition of particles does vary greatly and depends on numerous geographical, meteorological, and source-specific variables. Generally, environmental particles include inorganic components (sulfates, nitrates, ammonium, chloride, and trace metals), elemental and organic carbon, biological components (bacteria, spores, and pollens), and adsorbed volatile and semivolatile organic compounds [3]. In addition, environmental particles, when mixed with atmospheric gases (ozone, sulfur nitric oxides, and carbon monoxide) can generate environmental aerosols. Particles are usually defined as PM10 and PM2.5 with diameter less than 10 and 2.5 μm, respectively. Any fraction may have different effects; that is, PM with aerodynamic diameter less than 10 to 2.5 μm does generate a bigger amount of hydroxyl radical due to the heavy metals adsorbed on the pores and surfaces of the particles, whereas particles of larger size (PM10) deposit mainly in the upper airways and can be cleared by the mucociliary system [4, 5]. Recently, however, interest has also focused on the ultrafine particles (UFPs) with a diameter less than 100 nm; UFPs are considered important with respect to health effects because of their very high alveolar deposition fraction, large surface area, chemical composition, and ability to enter into the circulation and induce inflammation. Vehicle emissions, in particular related to diesel engines, diesel exhaust particles (DEPs), are a major source of environmental UFPs, which in the presence of poor ventilation may penetrate indoor, where additional sources including environmental tobacco smoke, cooking, burning of candles, and chemical reactions are present [6–10]. Long-term exposure to high levels of such particles can increase risk of cancer, respiratory diseases, and arteriosclerosis, whereas short-term exposure peaks can cause exacerbation of bronchitis, asthma, and other respiratory diseases as well as changes in heart-rate variability [2, 11–13]. The general consensus does indicate that the mechanism of air pollution-induced health effects involves an inflammation-related cascade and oxidation stress both in lung, vascular, and heart tissue [14–19]. Inflammation is initially a protective mechanism which removes the injurious stimuli and produces reactive oxygen species (ROS) able to induce cell killing. In the early phase of inflammation, oxidant stress does not directly cause cell damage and can induce the transcription of stress defense genes including antioxidant genes. This preconditioning effect of ROS enhances the resistance against future inflammatory oxidant stress and promotes the initiation of tissue repair processes. The additional release of cell contents amplifies the inflammatory process and consequently can induce tissue injury [20]. Oxidation damage has been implicated in many degenerative and nondegenerative diseases, including cardiovascular and pulmonary diseases, diabetes, and Alzheimer disease. Oxidation stress derived from an unbalance between ROS formation and individual antioxidant activity potentially does lead to damage of lipids, proteins, and macromolecules such as DNA and RNA [21]. This paper will focus on the mechanisms of oxidative stress induction and cellular damage by air pollution exposure on pulmonary and cardiovascular systems.2. Possible Mechanisms of Oxidative Stress Induced by Air Pollution ExposureIn the last decades, great attention has been paid to air pollution exposure due to vehicular traffic and other combustion processes. PM and gas pollutants are considered to be the most important factors in urban areas, and several mechanisms have been hypothesized to explain the adverse health effects in humans, especially in the cardiopulmonary system [22]. Although each air pollutant can exert its own specific toxicity in the respiratory and cardiovascular systems, ozone, oxides of nitrogen, and suspended particulates all share a common property of being potent oxidants, either through direct effects on lipids and proteins or indirectly through the activation of intracellular oxidant pathways [23–25].ROS can be generated from the surface of particles where polycyclic aromatic hydrocarbons (PAH) and nitro-PAH are adsorbed, other than transition metals (iron, copper, chromium, and vanadium) that catalyzing Fenton’s reaction (Fe2+ + H2O2 + H+ → Fe3+ + OH• + H2O) generate the highly reactive hydroxyl radical able to induce oxidative DNA damage [26, 27]. Several studies have shown that iron and other transition metals leaching from particles or by their presence on particle surfaces play a role in the generation of ROS in biological systems [28]. Particles bound benzo(a)pyrene has been shown to be bioavailable and can induce oxidative DNA damage in systemic target organs, including lung and kidney [29, 30]. Moreover, it should be noted that ozone and nitrogen dioxide are usually present together with particles in environmental air. They are also oxidants with potential effects in terms of oxidative DNA damage. Similarly, volatile compounds, such as benzene, in urban air pollution can induce DNA oxidation [31, 32]. In addition, photochemical oxidants (ozone and peroxyacetyl nitrate), secondary pollutants formed by the action of sunlight on an atmosphere that does contain reactive hydrocarbons and NOx, contribute to increase oxidation stress [33]. Then, in the presence of high ROS formation, mitochondrial damage with induction of NADPH- oxidase isoform 4 (NOX4) does occur, together with an activation of inflammatory cells (neutrophils, eosinophils, and monocytes) and increased numbers of macrophages capable of ROS and reactive nitrogen species generation [34–36]. Initially, when oxidative stress is relatively low, various transcription factors, such as the nuclear factor erythroid-2 (Nrf2), induce a series of antioxidant and detoxification enzymes (e.g., catalase, superoxide dismutase, and glutathione S-transferase) that counteract ROS formation protecting from adverse biological outcomes [37, 38]. In the second phase, if the protective antioxidant response fails or is inadequate to deal with increasing ROS production, the result is a proinflammatory situation with various cytotoxic effects [39]. These effects are mediated by the redox-sensitive mitogen-activated protein kinase (MAPK) and NF-κB cascades that are responsible for the expression of cytokines, chemokines, and adhesion molecules, which are involved in inflammatory processes [39].3. Atmospheric GasesGaseous pollutants contribute to a great extent in composition variations of the atmosphere and are mainly due to combustion of fossil fuels and to emission of motor vehicles [40].Ozone is a strong oxidizing agent formed in the troposphere through a complex series of reactions involving the action of sunlight in nitrogen dioxide and hydrocarbons. Ozone initiate intracellular oxidative stress through ozonide and hydroperoxide formation. This mechanism of oxidative damage involves the activation of Nrf2, heat shock protein 70, NF-κB, increased expression of a range of proinflammatory cytokines (TNFα and interleukin 1β), chemokines (e.g., interleukin 8), and adhesion genes; ozone is also an activator of protein-1 fos and c-jun onco genes [41, 42]. The major source of anthropogenic emissions of nitrogen oxides into the atmosphere is the combustion of fossil fuels deriving from stationary sources (heating, power generation) and motor vehicles. In environmental conditions, nitric oxide is rapidly transformed into nitrogen dioxide by atmospheric oxidants such as ozone [43].Various antioxidants, like ascorbic acid, uric acid, and thiols, act as powerful scavengers of O3 and NO2• radical in body fluids, likely protecting lung lining fluids against inhaled oxidizing air pollutants [44]. When such defense mechanisms are overwhelmed, O3 may injure the underlying cells by inducing lipid peroxidation and activating inflammatory gene expression [45]. In vitro and in vivo studies, both in animals and human beings, confirm the capacity of nitrogen dioxide to activate oxidant pathways although less potently than ozone [46]. Volatile organic compounds are a class of compounds which includes chemical species of organic nature such as benzene, but the majority of gaseous pollutants are inhaled and, therefore. mainly affect the respiratory and cardiovascular systems. Among gaseous pollutants, carbon monoxide (CO) has been described as one of the main pollutants responsible for the development of cardiovascular diseases [47], while benzene can also induce haematological problems and cancer [48]. Benzene is a commonly used industrial chemical and a constituent of gasoline [31]. Inhalation is the most important route of absorption during occupation-related exposure. Benzene toxicity is attributed to its metabolism, which does lead to the formation of reactive metabolites such as hydroquinone and its oxidized form benzoquinone which are highly reactive molecules and, by means of redox cycling, produce ROS [49]. Furthermore, the addition of antioxidant enzymes has been shown to block oxidative damage induced by the above-mentioned metabolites confirming the role of ROS production and oxidative stress in hydroquinone and benzoquinone cytotoxicity [50]. Uzma et al. [31] demonstrated that occupation-related exposure to benzene causes oxidative stress, immune suppression, and inducing the expression of tumour-suppressing gene p53 in gasoline filling workers. These authors hypothesized that the increase in the p53 expression may block the cell cycle at G1 phase and go on to repair DNA damage, which is the initial step in tumour suppression.4. Oxidative Stress from Organic FractionAmbient PM, does consist of complex and various mixtures of particles suspended in the breathing air [50]. Major sources of PM are factories, power plants, refuse incinerators, motor vehicles, building activity, fires, and natural windblown dust. The size of the particles vary, and there is strong evidence supporting that ultrafine and fine particles are more hazardous than larger ones in terms of mortality and cardiovascular and respiratory effects [51].Results from various surveys have demonstrated that oxidative potential of fine and ultrafine particles is the result of significant amounts of organic carbon compounds, such as quinones and PAHs. In the organic fraction originating in the air from incomplete combustion processes, the major reactive and toxic compounds are substituted (e.g., methyl naphthalene) and unsubstituted PAH, nitro-PAH (1-nitropyrene and 3-nitro-fluoranthene), dinitro-PAH (dinitro pyrene) and peroxyacetyl nitrate [52, 53]. Moreover, reactive intermediates in the oxidation of mixtures of volatile organic compounds (VOCs), oxides of nitrogen (NOx), hydroxy radical, and ozone are shown to play a central role in the formation and fate of airborne toxic chemicals, PAH, and fine particles [52]. The main pathways of metabolic activation of PAHs are generation of diol epoxides catalyzed by cytochrome P450 (CYP450), leading to DNA adduct formation, formation of radical cations catalyzed by CYP450 peroxidases, and formation of redox-active quinones [54]. Valavanidis et al. [55] demonstrated that redox-active transition metals, redox cycling quinoids and PAH act synergically to produce ROS. J. Y. C. Ma and J. K. H. Ma [56] reported that organic fraction of DEP, mainly constituent of PAH and quinones, does undergo to metabolic activation in the lung and liver of exposed animals, is able to induce CYP4501A1 isoform expression that generates ROS and reactive PAH-quinones. In addition, PM initiates inflammatory damage upregulating proinflammatory cytokines and chemokines; in vitro observations have shown that PM exposure may cause expression of nuclear factor NF-κB-related genes and oxidant-dependent NF-κB activation [57, 58]. To defend against oxidative damage, cells increase the production of antioxidant enzymes through the activation of the Nrf2, [37] and PM appears to inhibit protective enzymes involved in oxidative stress responses leading to the activation of additional intracellular signaling cascades that regulate the expression of cytokine and chemokine genes [59]. Many recent observations have shown that DEPs, because of their fine and ultrafine composition, play an important role on oxidative cellular damage through ROS generation causing lipid peroxidation and oxidative DNA damage. Some DEPs consist of a carbon core or organic droplets with adsorbed organic compounds, such as PAH, quinines, and redox-active metals. The capacity of DEPs to induce oxidative stress is largely related to these adsorbed components [60, 61].5. Oxidative Stress Induced by Transition MetalsTransition metals such as iron, lead, mercury, cadmium, silver, nickel, vanadium, chromium, manganese, and copper are detectable in PM2.5 and UFPs adsorbed on their surface and are capable of ROS formation by Fenton’s reaction [35]. As critical constituents of PM, transition metals were postulated to be involved in a number of pathological processes of the respiratory system through free radical-mediated damage [62]. They are natural components of the earth's crust and enter into the environment through a wide variety of sources, including combustion, waste water discharges, and manufacturing facilities. Iron is a well-known soot suppressant that might be emitted into the atmosphere in the form of ultrafine particles [63]. Zinc is a major metal element detected in traffic derived PM2.5, deriving from waste oil samples [64]. Copper is a component of car brake pads, however, ceramic brake pads contain 10%–20% copper by mass, while the metallic brake pads contained about 70% iron with very little copper. This metal in PM has also been linked to road traffic sources associated to PM2.5 [64]. Soluble metals in inhaled particles, such as Fe, Ni, V, Co, Cu, and Cr, were associated with increased ROS production, followed by cellular oxidative stress in airway epithelial cells [65].6. Air Pollution Induced-Oxidative Damage in Target Organs: Cardiovascular and Pulmonary Systems6.1. Cardiovascular SystemDiesel and gasoline vehicle emissions in the urban areas have dominant contributions to environmental particles, especially those located in the ultrafine range. Because of their small size and large surface area, UFPs have demonstrated unique biochemical characteristics, such as enhanced ability to adsorb or absorb organic molecules and to penetrate into cellular targets in the human pulmonary and cardiovascular systems [66, 67]. UFPs may be directly transported to the cardiac vasculature, where they can induce arrhythmias, reduce myocyte contractility, and decrease coronary blood flow [10, 68]. Studies by Brook et al. [69] demonstrated that fine particulate air pollution and ozone cause acute arterial vasoconstriction in healthy humans, while Urch et al. [70] reported that fine particles exposure pollution raise blood pressure and impair vascular function. In addition, UFP exposure depresses myocardial contractile response and coronary flow in both spontaneously hypertensive and wild-type rats [71], the same observation was found by Simkhovich et al. [72] in young and old rat hearts. Long-term exposure to low concentrations of PM2.5 has been shown to alter vasomotor tone, lead to vascular inflammation, and potentiate atherosclerosis induced by highly fat-containing chow in susceptible mice [73]. In addition, Suwa et al. [74] reported that exposure to PM10 cause progression of atherosclerotic lesions towards a more advanced phenotype hyperlipidemic rabbits. Moreover, atherosclerotic lesions of thoracic aorta were reported to be significantly increased with pronounced macrophage infiltration and lipid deposition in Apolipoprotein E (−/−) ApoE (−/−) mice exposed to PM2.5 through NADPH oxidase dependent pathways [75]. ApoE (−/−) mice exposed to ozone showed increased oxidative stress and mitochondrial DNA damage, decreased vascular endothelial nitric oxide synthase, and significantly increased atherogenesis compared to filtered air exposed controls [76]. Recently, Cherng et al. [77] reported that DEP exposure enhances vasoconstriction and diminishes acetylcholine-induced dilatation in coronary arteries of animals in a nitric oxide synthase-dependent manner. Baccarelli et al. [78] showed that air pollution is associated with changes in the global coagulation function, after short-term exposure to air pollution in normal subjects resident in Lombardia Region, Italy. Road traffic-related pollutants may increase a heart-rate-corrected QT interval among people with diabetes, obesity and nonsmoking elderly individuals and the number of genetic variants related to oxidative stress does increase this effect [79]. On the contrary, Mordukhovich et al. [80], despite the positive associations between blood pressure and black carbon, found no effects on gene variants related to antioxidative defense. Increases in black carbon and PM2.5 were associated with increases in blood pressure, heart-rate, endothelin-1, vascular endothelial growth factor, and oxidative stress markers and with a decrease in brachial artery diameter in nonsmoking seniors [81]. More recently, Kooter et al. [82] showed that diesel engine exhaust exposure induces a pulmonary antioxidant response, with an increased activity of the anti-oxidant enzymes glutathione peroxidase, superoxide dismutase, heme oxygenase-1 protein, heme oxygenase activity, and uric acid which precedes the inflammatory response (an increase in IL-6 and TNF-α) in rats. In addition, since the authors found that increased plasma thrombogenicity and antioxidant defense gene expression in aorta tissue shortly after the exposure does occur, they hypothesized a direct translocation of diesel engine exhaust components to the vasculature even if the mediation by other pathways cannot be excluded [82]. 6.2. PulmonaryA strong correlation has been found between PM concentration of redox-active compounds and damage in macrophages and bronchial epithelial cells [83–85]. Moreover, in human airway epithelial cells, organic compounds adsorbed on particle surfaces does promote inflammation through CYP1A1-mediated ROS generation and release of cytokines after activation of transduction pathways involving MAPK and the transcription factor NF-kappaB [86]. Recently, Andersson et al. [26] reported that 1-nitropyrene, one of the most abundant nitro-PAHs in diesel exhausts, induces DNA damage by ROS formation in human endothelial cells, and this effect was mainly mediated by metabolites mainly generating by reduction of nitro group, as it has been previously reported by Topinka et al. [87] in rat hepatocytes. Increased production of ROS after PM exposure is suggested by the finding that many of the proinflammatory genes (TNF-α and IL-8, among others) induced upon exposure to PM are regulated by redox sensitive transcription factors such as NF-κB, activator protein 1 (AP-1) and CAATT/enhancer binding protein (C/EBP). Activation of these transcription factors and increased transcription of downstream genes has been reported in human alveolar and bronchial epithelial cells in response to PM exposure [88–92]. Several studies have demonstrated that air pollution particles induce inflammatory mediator release and oxidative stress in lung epithelial cells and alveolar macrophages. When reaching the bone marrow [93], cytokines and chemokines released from the lung stimulate migration of neutrophils and their precursors into the circulation. In the short-term, there is acute tissue damage with activation of the epidermal-growth-factor receptor pathway, and evidence for organ-repair responses [94]. Vanadium pentoxide (V2O5) is a component of PM derived from fuel combustion as well as a source of occupation-related exposure in humans [95]. Sørensen et al. [95] indicate that vanadium and chromium (VI) detectable in PM(2.5) have an effect on oxidative DNA damage in human lymphocytes, after reduction to chromium (III) in the cells. Since, outdoor PM and urinary 1-hydroxypyrene (PAH exposure marker) were synergistically associated with urinary MDA levels of schoolchildren, Bae et al. [96] concluded that exposure to PM air pollution and PAH can induce oxidative stress in schoolchildren. In addition, these authors found that urinary MDA levels are also associated with some metals bound to PM10 and PM2.5 suggesting that metals bound to PM are responsible, at least in part, for the oxidative stress [96]. The oxidized species arising from the reaction between ozone and lining fluid are involved in the signaling cascade of inflammatory cells into the lung and contribute to the acute bronchoconstrictor response and hyperresponsiveness observed in asthma on exposure to this pollutant [97, 98]. Furthermore, has been reported that ozone is able to induce apoptosis, DNA damage, and cytotoxicity on human alveolar epithelial type I-like cells and in mice exposed to ozone for 6 weeks [99, 100]. While, Ferecatu et al. [84] reported an antiapoptotic effect of PAH adsorbed on PM2.5 that in addition to the well-documented inflammatory response may explain the persistence of a prolonged inflammation state induced after pollution exposure and might delay repair processes of injured tissues in primary cultures of human bronchial epithelial cells. Chirino et al. [101] found ROS generation and decreased glutathione and the activity of the antioxidant enzymes, such as superoxide dismutase and glutathione reductase, in a human lung epithelial cell line exposed to PM10. Recently, it has been found that bus drivers exposed to PAH and volatile compounds displayed a higher level of DNA instability and oxidative damage than the controls and the incidence of oxidized lesions in lymphocyte DNA correlated with exposure to benzene. Moreover, those of the drivers with at least one variant of 8-oxoguanine glycosylate 1 (hOGG1) (Cys/Cys or Ser/Cys) allele tended to have higher oxidative DNA damage in lymphocyte than those with the wild genotype [102]. In addition, in the same year Delfino et al. [103] reported that PM (ranged from 0.25 to 2.5 μm) and O3 were positively associated with exhaled nitrogen monoxide and that PM0.25, CO, and NO were positively associated with IL-6, while ROS were associated with both outcomes in elderly subjects enrolled.7. Defense Mechanisms against ROS FormationAntioxidants in the lung are the first line of defense against ROS [104]. The composition and quantity of antioxidants in respiratory tract lining fluids may represent an important determinant of individual responsiveness to air pollutants, but it should be thought of as a dynamic equilibrium with the antioxidant defenses within the epithelium and a more remote plasma pool [105]. Interestingly, the results obtained by Osburn and Kensler [106] demonstrated that the activation of transcriptional factor Nrf2 determines an upregulation of antioxidant enzymes that represents an adaptive response to face the exposure to oxidant pollutants providing a pivotal defense mechanism against environmental hazards, including various air pollutants. Successively, Rubio et al. [107] observed that Nrf2 does protect against benzene metabolites in human lung cells, and knockdown of Nrf2 greatly does enhance cytotoxicity and cell death associated with reduced glutathione levels and loss of inducibility of antioxidant response elements (ARE-driven) genes.Although the interrelation among antioxidant levels in the respiratory tract, cellular and plasma levels are not well understood, it appears that the susceptibility of the lung to oxidative injury depends largely on its ability to upregulate protective scavenging systems. A recent review by Rubio et al. [108] indicates that air pollutants are Nrf2 pathway inductors which regulate the expression of cytoprotective and detoxifying enzymes as well as antioxidants having an important role in the defense against atmospheric pollutant-induced toxicity.8. ConclusionsIn conclusion, several experimental and epidemiological studies have proved exposure to air pollution to be an important determinant of overall pulmonary and cardiovascular risk damage and possibly have an influence on traditional risk factors. Although each environmental pollutant has its own mechanism of toxicity, most pollutants, like UFP, PM2.5, ozone, nitrogen oxides, and transition metals, are potent oxidants or capable of ROS production. Consequently, the promotion of oxidative stress has been identified as one of the most important mechanisms responsible for toxic air pollutant effects. Oxidative stress can trigger redox-sensitive pathways that lead to different biological processes like inflammation and cell death. Recently, Environmental Pollution Agency (EPA) revised the level of the 24-h PM2.5 standard to 35 μg/m3, moved the 24-h PM10 standard from 75 at 150 μg/m3, and revoked the annual standard, because available evidence generally did not suggest a link between long-term exposure to current ambient levels of coarse particles and health or welfare effects [109]. However, a vast number of data indicate that in general, smaller size fraction, containing higher concentration of PAH, transition metal, and semiquinones, has a higher ROS capacity and consequently should be capable to induce severe toxicological effects. Thus, change in the composition of this PM are likely to modify its health impact. Road traffic is known to vastly contribute to PM exposure. Traffic intensity and quality should then be important determinants of the qualitative characteristics of PM spread in the atmosphere. In addition, although the interrelation between antioxidant levels in respiratory and cardiovascular systems, cellular and plasma levels is not yet well understood; it appears that the susceptibility of target organs to oxidative injury largely depends on cell ability to upregulate protective scavenging systems such as Nrf2. This transcription factor does regulate the expression of numerous cytoprotective genes that detoxify reactive species playing an important role in the defense against atmospheric pollutant-induced toxicity.However, many questions remain unanswered, but in the future, rapid developments in molecular biology, proteomics, and genomics will help to completely clarify the biological mechanisms involved in pulmonary and cardiovascular injuries caused by air pollution.AcknowledgmentThis work is supported by a fund of University of Florence.References
J. Schwartz, “Air pollution and daily mortality: a review and meta analysis,” Environmental Research, vol. 64, no. 1, pp. 36–52, 1994. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusD. W. Dockery, A. C. Pope III, X. Xu et al., “An association between air pollution and mortality in six U.S. cities,” New England Journal of Medicine, vol. 329, no. 94, pp. 1753–1759, 1993. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusR. M. Harrison and J. Yin, “Particulate matter in the atmosphere: which particle properties are important for its effects on health?” Science of the Total Environment, vol. 249, no. 1–3, pp. 85–101, 2000. View at Publisher · View at Google Scholar · View at Scopus Environmental Protection Agency, Air Quality Criteria for Particulate Matter, vol. III of EPA/600/P-95/001CF, National Center for Environmental Assessment, Research Triangle Park, NC, USA, 1996. J. Ferin, G. Oberdörster, and D. P. Penney, “Pulmonary retention of ultrafine and fine particles in rats,” American Journal of Respiratory Cell and Molecular Biology, vol. 6, no. 5, pp. 535–542, 1992. View at ScopusJ. Ferin, “Pulmonary retention and clearance of particles,” Toxicology Letters, vol. 72, no. 1–3, pp. 121–125, 1994. View at Publisher · View at Google Scholar · View at ScopusL. Kliucininkas, D. Martuzevicius, E. Krugly et al., “Indoor and outdoor concentrations of fine particles, particle-bound PAHs and volatile organic compounds in Kaunas, Lithuania,” Journal of Environmental Monitoring, vol. 13, no. 1, pp. 182–191, 2011. View at Publisher · View at Google Scholar · View at PubMedM. Dennekamp, S. Howarth, C. A. J. Dick, J. W. Cherrie, K. Donaldson, and A. Seaton, “Ultrafine particles and nitrogen oxides generated by gas and electric cooking,” Occupational and Environmental Medicine, vol. 58, no. 8, pp. 511–516, 2001. View at Publisher · View at Google Scholar · View at ScopusJ. I. Levy, T. Dumyahn, and J. D. Spengler, “Particulate matter and polycyclic aromatic hydrocarbon concentrations in indoor and outdoor microenvironments in Boston, Massachusetts,” Journal of Exposure Analysis and Environmental Epidemiology, vol. 12, no. 2, pp. 104–114, 2002. View at Publisher · View at Google Scholar · View at ScopusU. Franck, O. Herbarth, B. Wehner, A. Wiedensohler, and M. Manjarrez, “How do the indoor size distributions of airborne submicron and ultrafine particles in the absence of significant indoor sources depend on outdoor distributions?” Indoor Air, vol. 13, no. 2, pp. 174–181, 2003. View at Publisher · View at Google Scholar · View at ScopusB. Brunekreef and S. T. Holgate, “Air pollution and health,” The Lancet, vol. 360, no. 9341, pp. 1233–1242, 2002. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusA. Peters, D. W. Dockery, J. E. Muller, and M. A. Mittleman, “Increased particulate air pollution and the triggering of myocardial infarction,” Circulation, vol. 103, no. 23, pp. 2810–2815, 2001. View at ScopusY. J. Li, H. Takizawa, and T. Kawada, “Role of oxidative stresses induced by diesel exhaust particles in airway inflammation, allergy and asthma: their potential as a target of chemoprevention,” Inflammation and Allergy, vol. 9, no. 4, pp. 300–305, 2010. A. J. Ghio, C. Kim, and R. B. Devlin, “Concentrated ambient air particles induce mild pulmonary inflammation in healthy human volunteers,” American Journal of Respiratory and Critical Care Medicine, vol. 162, no. 3 I, pp. 981–988, 2001. View at ScopusK. Donaldson and V. Stone, “Current hypotheses on the mechanisms of toxicity of ultrafine particles,” Annali dell'Istituto Superiore di Sanita, vol. 39, no. 3, pp. 405–410, 2003. View at ScopusX. Y. Li, D. Brown, S. Smith, W. MacNee, and K. Donaldson, “Short-term inflammatory responses following intratracheal instillation of fine and ultrafine carbon black in rats,” Inhalation Toxicology, vol. 11, no. 8, pp. 709–731, 1999. View at ScopusY. Bai, A. K. Suzuki, and M. Sagai, “The cytotoxic effects of diesel exhaust particles on human pulmonary artery endothelial cells in vitro: role of active oxygen species,” Free Radical Biology and Medicine, vol. 30, no. 5, pp. 555–562, 2001. View at Publisher · View at Google Scholar · View at ScopusS. Hirano, A. Furuyama, E. Koike, and T. Kobayashi, “Oxidative-stress potency of organic extracts of diesel exhaust and urban fine particles in rat heart microvessel endothelial cells,” Toxicology, vol. 187, no. 2-3, pp. 161–170, 2003. View at Publisher · View at Google Scholar · View at ScopusL. E. Wold, B. Z. Simkhovich, M. T. Kleinman et al., “In vivo and in vitro models to test the hypothesis of particle-induced effects on cardiac function and arrhythmias,” Cardiovascular Toxicology, vol. 6, no. 1, pp. 69–78, 2006. View at Publisher · View at Google Scholar · View at ScopusH. Jaeschke, “Reactive oxygen and mechanisms of inflammatory liver injury: present concepts,” Journal of Gastroenterology and Hepatology, vol. 26, no. 1, pp. 173–179, 2011. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusL. Risom, P. Møller, and S. Loft, “Oxidative stress-induced DNA damage by particulate air pollution,” Mutation Research, vol. 592, no. 1-2, pp. 119–137, 2005. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusJ. Schnelle-Kreis, U. Küpper, M. Sklorz et al., “Daily measurement of organic compounds in ambient particulate matter in Augsburg, Germany: new aspects on aerosol sources and aerosol related health effects,” Biomarkers, vol. 14, no. 1, pp. 39–44, 2009. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusP. MØller and S. Loft, “Oxidative damage to DNA and lipids as biomarkers of exposure to air pollution,” Environmental Health Perspectives, vol. 118, no. 8, pp. 1126–1136, 2010. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusA. Valavanidis, K. Fiotakis, and T. Vlachogianni, “Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms,” Journal of Environmental Science and Health C, vol. 26, no. 4, pp. 339–362, 2008. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusH. A. Jeng, “Chemical composition of ambient particulate matter and redox activity,” American Journal of Physiology Lung Cell Molecular Physiology, vol. 293, pp. 170–181, 2007. H. Andersson, E. Piras, J. Demma, and B. Hellman, “Low levels of the air pollutant 1-nitropyrene induce DNA damage, increased levels of reactive oxygen species and endoplasmic reticulum stress in human endothelial cells,” Toxicology, vol. 262, no. 1, pp. 57–64, 2009. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusJ. H. Park, A. B. Troxel, R. G. Harvey, and T. M. Penning, “Polycyclic aromatic hydrocarbon (PAH) o-quinones produced by the Aldo-Keto-Reductases (AKRs) generate abasic sites, oxidized pyrimidines, and 8-Oxo-dGuo via reactive oxygen species,” Chemical Research in Toxicology, vol. 19, no. 5, pp. 719–728, 2006. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusA. J. Ghio, J. H. Richards, J. D. Carter, and M. C. Madden, “Accumulation of iron in the rat lung after tracheal instillation of diesel particles,” Toxicologic Pathology, vol. 28, no. 4, pp. 619–627, 2000. View at ScopusP. Gerde, B. A. Muggenburg, M. Lundborg, Y. Tesfaigzi, and A. R. Dahl, “Respiratory epithelial penetration and clearance of particle-borne benzo[a]pyrene,” Research Report, vol. 101, pp. 5–27, 2001. View at ScopusK. B. Kim and B. M. Lee, “Oxidative stress to DNA, protein, and antioxidant enzymes (superoxide dismutase and catalase) in rats treated with benzo(a)pyrene,” Cancer Letters, vol. 113, no. 1-2, pp. 205–212, 1997. View at Publisher · View at Google Scholar · View at ScopusN. Uzma, S. S. Kumar, and M. A. H. Hazari, “Exposure to benzene induces oxidative stress, alters the immune response and expression of p53 in gasoline filling workers,” American Journal of Industrial Medicine, vol. 53, no. 12, pp. 1264–1270, 2010. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusM. Sørensen, H. Skov, H. Autrup, O. Hertel, and S. Loft, “Urban benzene exposure and oxidative DNA damage,” Science of the Total Environment, vol. 309, no. 1–3, pp. 69–80, 2003. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusH. H. Liu, Y. C. Wu, and H. L. Chen, “Production of ozone and reactive oxygen species after welding,” Archives of Environmental Contamination and Toxicology, vol. 53, no. 4, pp. 513–518, 2007. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusA. Baulig, M. Garlatti, V. Bonvallot et al., “Involvement of reactive oxygen species in the metabolic pathways triggered by diesel exhaust particles in human airway epithelial cells,” American Journal of Physiology, vol. 285, no. 3, pp. L671–L679, 2003. View at ScopusN. Li, C. Sioutas, A. Cho et al., “Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage,” Environmental Health Perspectives, vol. 111, no. 4, pp. 455–460, 2003. View at ScopusN. Amara, R. Bachoual, M. Desmard et al., “Diesel exhaust particles induce matrix metalloprotease-1 in human lung epithelial cells via a NADP(H) oxidase/NOX4 redox-dependent mechanism,” American Journal of Physiology, vol. 293, no. 1, pp. L170–L181, 2007. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusK. W. Kang, S. J. Lee, and S. G. Kim, “Molecular mechanism of Nrf2 activation by oxidative stress,” Antioxidants and Redox Signaling, vol. 7, no. 11-12, pp. 1664–1673, 2005. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusJ. D. Hayes and M. McMahon, “NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer,” Trends in Biochemical Sciences, vol. 34, no. 4, pp. 176–188, 2009. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusA. H. Sprague and R. A. Khalil, “Inflammatory cytokines in vascular dysfunction and vascular disease,” Biochemical Pharmacology, vol. 78, no. 6, pp. 539–552, 2009. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusK. Katsouyanni, “Ambient air pollution and health,” British Medical Bulletin, vol. 68, pp. 143–156, 2003. View at Publisher · View at Google Scholar · View at ScopusB. G. Nichols, J. S. Woods, D. L. Luchtel, J. Corral, and J. Q. Koenig, “Effects of ozone exposure on nuclear factor-κB activation and tumor necrosis factro-α expression in human nasal epithelial cells,” Toxicological Sciences, vol. 60, no. 2, pp. 356–362, 2001. View at Publisher · View at Google Scholar · View at ScopusD. Bassett, C. Elbon-Copp, S. Otterbein, H. Barraclough-Mitchell, M. DeLorme, and H. Yang, “Inflammatory cell availability affects ozone-induced lung damage,” Journal of Toxicology and Environmental Health A, vol. 64, no. 7, pp. 547–565, 2001. View at Publisher · View at Google Scholar · View at PubMed · View at Scopus WHO, “Air quality guidelines: for Europe,” World Health Organization Regional Publications, no. 91, pp. 1–287, 2001. View at ScopusW. Yang and S. T. Omaye, “Air pollutants, oxidative stress and human health,” Mutation Research, vol. 674, no. 1-2, pp. 45–54, 2009. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusB. Kosmider, J. E. Loader, R. C. Murphy, and R. J. Mason, “Apoptosis induced by ozone and oxysterols in human alveolar epithelial cells,” Free Radical Biology and Medicine, vol. 48, no. 11, pp. 1513–1524, 2010. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusH. Bayram, R. J. Sapsford, M. M. Abdelaziz, and O. A. Khair, “Effect of ozone and nitrogen dioxide on the release of proinflammatory mediators from bronchial epithelial cells of nonatopic nonasthmatic subjects and atopic asthmatic patients in vitro,” Journal of Allergy and Clinical Immunology, vol. 107, no. 2, pp. 287–294, 2001. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusM. L. Bell, R. D. Peng, F. Dominici, and J. M. Samet, “Emergency hospital admissions for cardiovascular diseases and ambient levels of carbon monoxide results for 126 united states urban counties,” Circulation, vol. 120, no. 11, pp. 949–955, 2009. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusG. Barreto, D. Madureira, F. Capani, L. Aon-Bertolino, E. Saraceno, and L. D. Alvarez-Giraldez, “The role of catechols and free radicals in benzene toxicity: an oxidative DNA damage pathway,” Environmental and Molecular Mutagenesis, vol. 50, no. 9, pp. 771–780, 2009. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusJ. L. Bolton, M. A. Trush, T. M. Penning, G. Dryhurst, and T. J. Monks, “Role of quinones in toxicology,” Chemical Research in Toxicology, vol. 13, no. 3, pp. 135–160, 2000. View at Publisher · View at Google Scholar · View at ScopusU. Pöschl, “Atmospheric aerosols: composition, transformation, climate and health effects,” Angewandte Chemie, vol. 44, no. 46, pp. 7520–7540, 2005. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusE. Boldo, C. Linares, J. Lumbreras et al., “Health impact assessment of a reduction in ambient PM(2.5) levels in Spain,” Environment International, vol. 37, no. 2, pp. 342–348, 2011. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusG. L. Squadrito, R. Cueto, B. Dellinger, and W. A. Pryor, “Quinoid redox cycling as a mechanism for sustained free radical generation by inhaled airborne particulate matter,” Free Radical Biology and Medicine, vol. 31, no. 9, pp. 1132–1138, 2001. View at Publisher · View at Google Scholar · View at ScopusB. J. Finlayson-Pitts Jr. and J. N. Pitts, “Tropospheric air pollution: ozone, airborne toxics, polycyclic aromatic hydrocarbons, and particles,” Science, vol. 276, no. 5315, pp. 1045–1052, 1997. View at Publisher · View at Google Scholar · View at ScopusV. Bonvallot, A. Baeza-Squiban, A. Baulig et al., “Organic compounds from diesel exhaust particles elicit a proinflammatory response in human airway epithelial cells and induce cytochrome p450 1A1 expression,” American Journal of Respiratory Cell and Molecular Biology, vol. 25, no. 4, pp. 515–521, 2001. View at ScopusA. Valavanidis, K. Fiotakis, E. Bakeas, and T. Vlahogianni, “Electron paramagnetic resonance study of the generation of reactive oxygen species catalysed by transition metals and quinoid redox cycling by inhalable ambient particulate matter,” Redox Report, vol. 10, no. 1, pp. 37–51, 2005. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusJ. Y. C. Ma and J. K. H. Ma, “The dual effect of the particulate and organic components of diesel exhaust particles on the alteration of pulmonary immune/inflammatory responses and metabolic enzymes,” Journal of Environmental Science and Health C, vol. 20, no. 2, pp. 117–147, 2002. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusA. Shukla, C. Timblin, K. BeruBe et al., “Inhaled particulate matter causes expression of nuclear factor (NF)-κB-related genes and oxidant-dependent NF-κB activation in vitro,” American Journal of Respiratory Cell and Molecular Biology, vol. 23, no. 2, pp. 182–187, 2000. View at ScopusR. Li, Z. Ning, R. Majumdar et al., “Ultrafine particles from diesel vehicle emissions at different driving cycles induce differential vascular pro-inflammatory responses: implication of chemical components and NF-κB signaling,” Particle and Fibre Toxicology, vol. 7, no. 1, pp. 6–18, 2010. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusR. Li, Z. Ning, J. Cui et al., “Ultrafine particles from diesel engines induce vascular oxidative stress via JNK activation,” Free Radical Biology and Medicine, vol. 15, no. 46, pp. 775–782, 2009. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusK. S. Kouassi, S. Billet, G. Garçon et al., “Oxidative damage induced in A549 cells by physically and chemically characterized air particulate matter (PM2.5) collected in Abidjan, Côte d'Ivoire,” Journal of Applied Toxicology, vol. 30, no. 4, pp. 310–320, 2010. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusA. Saxon and D. Diaz-Sanchez, “Air pollution and allergy: you are what you breathe,” Nature Immunology, vol. 6, no. 3, pp. 223–226, 2005. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusE. Vidrio, H. Jung, and C. Anastasio, “Generation of hydroxyl radicals from dissolved transition metals in surrogate lung fluid solutions,” Atmospheric Environment, vol. 42, no. 18, pp. 4369–4379, 2008. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusP. J. Schaumann, J. Borm, A. Herbrich et al., “Metal-rich ambient particles (particulate matter2.5) cause airway inflammation in healthy subjects,” American Journal of Respiratory and Critical Care Medicine, vol. 170, no. 8, pp. 898–903, 2004. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusM. E. Gerlofs-Nijland, J. A. M. A. Dormans, H. J. T. Bloemen et al., “Toxicity of coarse and fine particulate matter from sites with contrasting traffic profiles,” Inhalation Toxicology, vol. 19, no. 13, pp. 1055–1069, 2007. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusA. M. Knaapen, T. Shi, P. J. A. Borm, and R. P. F. Schins, “Soluble metals as well as the insoluble particle fraction are involved in cellular DNA damage induced by particulate matter,” Molecular and Cellular Biochemistry, vol. 234-235, pp. 317–326, 2002. View at Publisher · View at Google Scholar · View at ScopusA. Peters, H. E. Wichmann, T. Tuch, J. Heinrich, and J. Heyder, “Respiratory effects are associated with the number of ultrafine particles,” American Journal of Respiratory and Critical Care Medicine, vol. 155, no. 4, pp. 1376–1383, 1997. View at ScopusC. A. Pope, J. B. Muhlestein, H. T. May, D. G. Renlund, J. L. Anderson, and B. D. Horne, “Ischemic heart disease events triggered by short-term exposure to fine particulate air pollution,” Circulation, vol. 114, no. 23, pp. 2443–2448, 2006. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusR. J. Delfino, C. Sioutas, and S. Malik, “Potential role of ultrafine particles in associations between airborne particle mass and cardiovascular health,” Environmental Health Perspectives, vol. 113, no. 8, pp. 934–946, 2005. View at Publisher · View at Google Scholar · View at ScopusR. D. Brook, J. R. Brook, B. Urch, R. Vincent, S. Rajagopalan, and F. Silverman, “Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults,” Circulation, vol. 105, no. 13, pp. 1534–1536, 2002. View at Publisher · View at Google Scholar · View at ScopusB. Urch, F. Silverman, P. Corey et al., “Acute blood pressure responses in healthy adults during controlled air pollution exposures,” Environmental Health Perspectives, vol. 113, no. 8, pp. 1052–1055, 2005. View at Publisher · View at Google Scholar · View at ScopusH. Hwang, R. A. Kloner, M. T. Kleinman, and B. Z. Simkhovich, “Direct and acute cardiotoxic effects of ultrafine air pollutants in spontaneously hypertensive rats and Wistar-Kyoto rats,” Journal of Cardiovascular Pharmacology and Therapeutics, vol. 13, no. 3, pp. 189–198, 2008. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusB. Z. Simkhovich, P. Marjoram, M. T. Kleinman, and R. A. Kloner, “Direct and acute cardiotoxicity of ultrafine particles in young adult and old rat hearts,” Basic Research in Cardiology, vol. 102, no. 6, pp. 467–475, 2006. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusQ. Sun, A. Wang, X. Jin et al., “Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model,” Journal of the American Medical Association, vol. 294, no. 23, pp. 3003–3010, 2005. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusT. Suwa, J. C. Hogg, K. B. Quinlan, A. Ohgami, R. Vincent, and S. F. Van Eeden, “Particulate air pollution induces progression of atherosclerosis,” Journal of the American College of Cardiology, vol. 39, no. 6, pp. 935–942, 2002. View at Publisher · View at Google Scholar · View at ScopusG. C. Chuang, Z. Yang, D. G. Westbrook et al., “Pulmonary ozone exposure induces vascular dysfunction, mitochondrial damage, and atherogenesis,” American Journal of Physiology, vol. 297, no. 2, pp. L209–L216, 2009. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusM. J. Campen, A. K. Lund, T. L. Knuckles et al., “Inhaled diesel emissions alter atherosclerotic plaque composition in ApoE(-/-) mice,” Toxicology and Applied Pharmacology, vol. 242, no. 3, pp. 310–317, 2010. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusT. W. Cherng, M. L. Paffett, O. Jackson-Weaver, M. J. Campen, B. R. Walker, and N. L. Kanagy, “Mechanisms of diesel-induced endothelial nitric oxide synthase dysfunction in coronary arterioles,” Environmental Health Perspectives, vol. 119, no. 1, pp. 98–103, 2011. View at Publisher · View at Google Scholar · View at PubMedA. Baccarelli, A. Zanobetti, I. Martinelli et al., “Effects of exposure to air pollution on blood coagulation,” Journal of Thrombosis and Haemostasis, vol. 5, no. 2, pp. 252–260, 2007. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusE. S. Baja, J. D. Schwartz, G. A. Wellenius et al., “Traffic-related air pollution and QT interval: modification by diabetes, obesity, and oxidative stress gene polymorphisms in the normative aging study,” Environmental Health Perspectives, vol. 118, no. 6, pp. 840–846, 2010. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusI. Mordukhovich, E. Wilker, H. Suh et al., “Black carbon exposure, oxidative stress genes, and blood pressure in a repeated-measures study,” Environmental Health Perspectives, vol. 117, no. 11, pp. 1767–1772, 2009. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusL. Liu, T. Ruddy, M. Dalipaj et al., “Effects of indoor, outdoor, and personal exposure to particulate air pollution on cardiovascular physiology and systemic mediators in seniors,” Journal of Occupational and Environmental Medicine, vol. 51, no. 9, pp. 1088–1098, 2009. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusI. M. Kooter, M. E. Gerlofs-Nijland, A. J. Boere, et al., “Diesel engine exhaust initiates a sequence of pulmonary and cardiovascular effects in rats,” Environmental Health Perspectives, vol. 118, no. 8, pp. 1126–1136, 2010. L. J. den Hartigh, M. W. Lamé, W. Ham, M. J. Kleeman, F. Tablin, and D. W. Wilson, “Endotoxin and polycyclic aromatic hydrocarbons in ambient fine particulate matter from Fresno, California initiate human monocyte inflammatory responses mediated by reactive oxygen species,” Toxicology in Vitro, vol. 24, no. 7, pp. 1993–2002, 2010. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusI. Ferecatu, M. C. Borot, C. Bossard et al., “Polycyclic aromatic hydrocarbon components contribute to the mitochondria-antiapoptotic effect of fine particulate matter on human bronchial epithelial cells via the aryl hydrocarbon receptor,” Particle and Fibre Toxicology, vol. 7, no. 1, pp. 18–32, 2010. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusA. Baulig, M. Garlatti, V. Bonvallot et al., “Involvement of reactive oxygen species in the metabolic pathways triggered by diesel exhaust particles in human airway epithelial cells,” American Journal of Physiology, vol. 285, no. 3, pp. L671–L679, 2003. View at ScopusA. Churg, C. Xie, X. Wang, R. Vincent, and R. D. Wang, “Air pollution particles activate NF-κB on contact with airway epithelial cell surfaces,” Toxicology and Applied Pharmacology, vol. 208, no. 1, pp. 37–45, 2005. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusJ. Topinka, L. R. Schwarz, F. Kiefer et al., “DNA adduct formation in mammalian cell cultures by polycyclic aromatic hydrocarbons (PAH) and nitro-PAH in coke oven emission extract,” Mutation Research, vol. 419, no. 1–3, pp. 91–105, 1998. View at Publisher · View at Google Scholar · View at ScopusL. A. Jimenez, J. Thompson, D. A. Brown et al., “Activation of NF-κB by PM10 occurs via an iron-mediated mechanism in the absence of IκB degradation,” Toxicology and Applied Pharmacology, vol. 15, no. 166, pp. 101–110, 2000. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusS. Becker, L. A. Dailey, J. M. Soukup, S. C. Grambow, R. B. Devlin, and Y. C. T. Huang, “Seasonal variations in air pollution particle-induced inflammatory mediator release and oxidative stress,” Environmental Health Perspectives, vol. 113, no. 8, pp. 1032–1038, 2005. View at Publisher · View at Google Scholar · View at ScopusS. Becker, S. Mundandhara, R. B. Devlin, and M. Madden, “Regulation of cytokine production in human alveolar macrophages and airway epithelial cells in response to ambient air pollution particles: further mechanistic studies,” Toxicology and Applied Pharmacology, vol. 207, no. 2, supplement, pp. S269–S275, 2005. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusS. S. Salvi, C. Nordenhall, A. Blomberg et al., “Acute exposure to diesel exhaust increases IL-8 and GRO-α production in healthy human airways,” American Journal of Respiratory and Critical Care Medicine, vol. 161, no. 2 I, pp. 550–557, 2000. View at ScopusE. D. Karoly, Z. Li, X. Hyseni, and Y. C. T. Huang, “Up-regulation of tissue factor in human pulmonary artery endothelial cells after ultrafine particle exposure,” Environmental Health Perspectives, vol. 115, no. 4, pp. 535–540, 2007. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusH. Ishii, S. Hayashi, J. C. Hogg et al., “Alveolar macrophage-epithelial cell interaction following exposure to atmospheric particles induces the release of mediators involved in monocyte mobilization and recruitment,” Respiratory Research, vol. 6, pp. 87–99, 2005. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusG. Oberdörster, “Significance of particle parameters in the evaluation of exposure-dose-response relationships of inhaled particles,” Inhalation Toxicology, vol. 8, pp. 73–89, 1996. View at ScopusM. Sørensen, R. P. F. Schins, O. Hertel, and S. Loft, “Transition metals in personal samples of PM2.5 and oxidative stress in human volunteers,” Cancer Epidemiology Biomarkers and Prevention, vol. 14, no. 5, pp. 1340–1343, 2005. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusS. Bae, X. C. Pan, S. Y. Kim et al., “Exposures to particulate matter and polycyclic aromatic hydrocarbons and oxidative stress in schoolchildren,” Environmental Health Perspectives, vol. 118, no. 4, pp. 579–583, 2010. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusH. Song, W. Tan, and X. Zhang, “Ozone induces inflammation in bronchial epithelial cells,” Journal of Asthma, vol. 48, no. 1, pp. 79–83, 2011. View at Publisher · View at Google Scholar · View at PubMedI. S. Mudway and F. J. Kelly, “Ozone and the lung: a sensitive issue,” Molecular Aspects of Medicine, vol. 21, no. 1–2, pp. 1–48, 2000. View at Publisher · View at Google Scholar · View at ScopusB. Kosmider, J. E. Loader, R. C. Murphy, and R. J. Mason, “Apoptosis induced by ozone and oxysterols in human alveolar epithelial cells,” Free Radical Biology and Medicine, vol. 48, no. 11, pp. 1513–1524, 2010. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusK. Triantaphyllopoulos, F. Hussain, M. Pinart et al., “A model of chronic inflammation and pulmonary emphysema after multiple ozone exposures in mice,” American Journal of Physiology, vol. 300, no. 5, pp. L691–L700, 2011. View at Publisher · View at Google Scholar · View at PubMedY. I. Chirino, Y. Sánchez-Pérez, Á. R. Osornio-Vargas et al., “PM10 impairs the antioxidant defense system and exacerbates oxidative stress driven cell death,” Toxicology Letters, vol. 193, no. 3, pp. 209–216, 2010. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusY. Bagryantseva, B. Novotna, P. Rossner et al., “Oxidative damage to biological macromolecules in Prague bus drivers and garagemen: impact of air pollution and genetic polymorphisms,” Toxicology Letters, vol. 199, no. 1, pp. 60–68, 2010. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusR. J. Delfino, N. Staimer, T. Tjoa et al., “Associations of primary and secondary organic aerosols with airway and systemic inflammation in an elderly panel cohort,” Epidemiology, vol. 21, no. 6, pp. 892–902, 2010. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusF. J. Kelly and T. D. Tetley, “Nitrogen dioxide depletes uric acid and ascorbic acid but not glutathione from lung lining fluid,” Biochemical Journal, vol. 325, no. 1, pp. 95–99, 1997. View at ScopusA. F. Behndig, I. S. Mudway, J. L. Brown et al., “Airway antioxidant and inflammatory responses to diesel exhaust exposure in healthy humans,” European Respiratory Journal, vol. 27, no. 2, pp. 359–365, 2006. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusW. O. Osburn and T. W. Kensler, “Nrf2 signaling: an adaptive response pathway for protection against environmental toxic insults,” Mutation Research, vol. 659, no. 1-2, pp. 31–39, 2008. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusV. Rubio, J. Zhang, M. Valverde, E. Rojas, and Z. Z. Shi, “Essential role of Nrf2 in protection against hydroquinone- and benzoquinone-induced cytotoxicity,” Toxicology in Vitro, vol. 25, no. 2, pp. 521–529, 2010. View at Publisher · View at Google Scholar · View at PubMed · View at ScopusV. Rubio, M. Valverde, and E. Rojas, “Effects of atmospheric pollutants on the Nrf2 survival pathway,” Environmental Science and Pollution Research, vol. 17, no. 2, pp. 369–382, 2010. View at Publisher · View at Google Scholar · View at PubMed · View at Scopus EPA, “Integrated science assessment for particulate matter,” Provisional Draft (Personal communication), 2008.