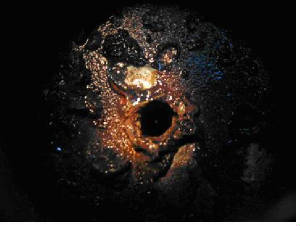
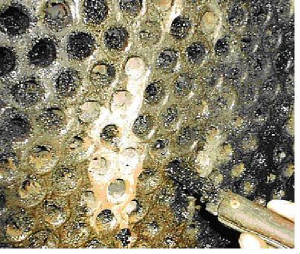
The major problems associated with any cooling water system are corrosion, scaling, fouling and microbial growth.
![Cooling Water Systems - Problems Cooling Water Systems - Problems]()
The principal objective of a good cooling water treatment programme is to prevent corrosion to extend equipment life, inhibit scale and deposit build up to maintain heat transfer across the exchangers/condensers and control the growth of micro-organisms which can both-corrode and foul the system. The objective is also to permit extended operation of the plant without necessitating any shut down due to water related problems. In today’s competitive scenario it is also necessary to continuously provide value addition and reduce the total cost of operation while fulfilling the above mentioned objectives, and it is here that many treatment programmes are found wanting.
On many occasions a water treatment vendor is selected based on the lowest cost. As with any industry, competition is also fierce in the water treatment segment and when the lowest quote is provided it is often only the basic requirements that are provided. The fine balance between cost and performance limits the flexibility of the treatment programme and operation, which is essential for value added programmes and services. Correct technical water treatment solution at the right price creates a WIN-WIN situation for both the plant and the water treatment company.
Value addition and reduction in the cost of operation can be realized when both work as true partners. After all the plant personnel know the plant best and the water treatment company knows their chemicals and programmes best. The two then have to harness their resources and work together to get the best out of any treatment programme. This approach brings in an atmosphere of trust, and transparency automatically follows paving the way for ‘Ethical Proposals’ of value for money without shortchanging either the equipment or performance.
The critical step in value added services is to conduct an audit. This is not to be construed as a compliance audit but should be purely a fact finding procedure. Only when a closer look is taken can areas of improvement be identified. The audit exercise should be broad based and the process of achieving them multidimensional to cover all angles of operations to arrive at the right conclusions and implementation of best practices within the operational constraints. The aim should be to maximize the returns on the investment that the plant makes by selecting a water treatment company as their partner in water management. This is what we call the ‘IPO’ of a cooling water treatment audit – Intent, Procedure and Output.
The intent of any cooling water treatment audit is three fold:
• MEASURE THE EFFECTIVENESS
• IMPROVE UPON THE PRACTICES
• INSTITUTE AND STANDARDIZE IMPROVEMENTS
In order to carry out the audit the following procedure is suggested:
A. Review the current conditions and service standardsThis is the starting point and forms the baseline data. Here, one can collect the following;
• Details of the treatment programme currently used.
• Overall treatment performance currently achieved and history.
• History of critical equipment and parameters.
• Critical process data relevant to water treatment.
• Specific contaminants, if present.
B.Collect system information in depthThe system and operational details are then gathered along with the water flow chart. Storage and pretreatment data , stagnant zones, Cooling tower structural information, seasonal wind direction and nearby equipment that can alter or influence the water parameters are some areas that can often provide valuable inputs for overall improvement.
C. Gather records, data and statisticsThe data for screening could include;
• Daily log sheets at operating levels
• Daily water analysis data
• The water flow diagrams
• Flow mapping data
• Monitoring data and records
• Data on microbial analysis
• Data on corrosion rates measured
• Exchanger monitoring data
• Various sample and product analysis
• Consumption of chemicals
• Specific observations made by the people on site
• Seasonal variations
D. Evaluate programme performanceThe programme performance evaluation is generally based on:
a. Corrosion control
b. Microbial control
c. Scaling & deposition control
d. General water chemistry
e. Heat exchanger behavior pattern
f. Heat exchanger inspection reports
E. Conduct site interviews/special studiesA very important and integral part of any audit is site interviews with key operating people at all levels. While providing important feedback on the present condition and performance, these interviews go a long way in providing critical information on the concerns and needs of the plant and also highlight operational limitations, if any.
Special studies could be diagnostic or analytical to arm us with more information or reconfirm existing data that can be beneficially used.
F. Measure costs: Actual Vs. capabilityThis data will allow us to optimize the feed rates of various chemicals and fine tune their frequency of addition to bring in direct benefits to the plant. Variations in the regular maintenance level can be reduced and the chemicals can be maintained in a narrower band of operation to avoid wide fluctuations and overfeed of chemicals.
G. Overall review and presentationOnce all the information is collated a detailed study of the data is necessary to suggest any improvements with regard to the system, programme and/or services to bring in value addition and cost benefit which is the sole objective of such an audit. The findings and suggestions should then be logically presented to the key officials of the unit who can then assist in the implementations of the suggested improvements.
OUTPUTThe whole purpose of an audit is the Pay Off. Some the pay offs could be;
• Optimisation of the treatment programme
• Improvement of the treatment performance
• Operation at higher stress conditions to save water
• Use discharge of other areas that would normally drain to the effluent as make up
• Effluent recycle
• Removal of bottlenecks to improve plant load (productivity)
AUDIT – Summary and Check list:As a summary, provided below is the audit check list in assisting a cooling water audit. This is a typical audit check list and depending on the system and the need it can be modified to suit individual requirement.
| Audit Summary – Checklist |
| 1 | Current Conditions and Service Review |
| a. | Treatment programme details |
| b. | General observations |
| c. | Corrosion observations |
| d. | Scaling observations |
| e. | Fouling observations |
| f. | Microbiology observations |
| 2 | System Information |
| a. | System details |
| b. | Hx details |
| c. | Plant location details |
| 3 | Records, Data and Statistics |
| a. | General observations & comments |
| 4 | Programme Performance Evaluation |
| a. | Corrosion control |
| b. | Microbial control |
| c. | Scale & deposit control |
| d. | General water chemistry |
| e. | Hx behavior pattern |
| 5 | Site Interviews |
| 6 | Special studies |
| a. | Diagnostic |
| b. | Microbial |
| c. | Analytical |
| 7 | Costs; Actual vs Design |
| 8 | Overall Review & Recommendation |
| a. | System |
| b. | Programme |
| c. | Service |
AUDIT Findings: ExamplesExample 1: A chemical plant in North India used river water for all their utilities. The river water was transported over 17kms and stored. The water was chlorinated in the plant battery limit before use. However SRB attack was still observed in the system and was particularly high in the transport pipelines. After detailed investigation it was found that a distillery existed upstream of the river from were the water was pumped to site and the waste water from the distillery was the cause of high microbial activity. Chlorination was started in the intake well in the river itself to get over the problem.
Example 2: Two plants located at a distance from each other produced the same product. They had identical technology and the plants were also identical in all respects. Both the plants had identical treatment programme. However one plant faced severe nitrifying bacteria problem and the other had severe sulfate reducing bacteria problem. Detailed audit helped in overcoming the problems at both sites. It was found that during certain periods the wind direction inoculated the cooling water systems with soil from the surrounding area.
One plant had an agricultural surrounding (Use of nitrogenous fertilizers) which brought nitrifying bacteria into the system. The other plant had many industries including sugar factories around it and that contributed towards SRB ingress into the system. The problems were addressed and resolved with specific microbial control regime.
Example 3: A unit in South-West India had three cooling towers at site. Similar programmes were applied in all the three cooling water systems. However it was found that while two systems yielded good treatment performance (CR 1-2 mpy), the corrosion rate in the third tower was always very high (8-12 mpy). This was initially baffling as the water chemistry and COC were identical. Infact the COC in the third tower was usually higher which should have reduced the corrosion potential when compared with the other towers. Initially it was thought that the sulfur dioxide from the surrounding stacks contributed to higher SRB leading to higher corrosion rate.
Specific biocide at increased frequency yielded only marginal benefit. After a detailed audit along with extensive lab and field studies at site it was found that the bulk return water temperature was very high (48 to 52 deg C) as compared to the other towers where it was normal. Initially the problem was solved by modifying the chemical treatment programme. Subsequently as a permanent measure an additional cell was constructed and attached to the system. After this the regular treatment programme as implemented in the other towers, provided similar good performance in the system.
Example 4: A chemical plant in Eastern India had a treatment programme that was yielding good treatment performance in all respects. However it was found that one particular high temperature condenser was becoming a bottleneck and it needed to be frequently cleaned. Due to high temperature the scales were predominantly calcium phosphate. After detailed studies and calculations and in partnership with the plant’s technical cell it was decided to convert the exchanger to two pass. This was a mechanical solution which solved the problem, as no deposit control agent would have provided any relief at those high temperatures.
Example 5: After a long good run, rapid deterioration was observed in the performance of a turbine condenser in a petrochemical plant. Further deterioration could result in a plant shutdown. The need of the hour was to quickly find the cause of the problem and rectify it. Initially it was suspected that scaling could be the problem which would necessitate on line cleaning, a tedious process. Observation of the other exchangers and the HTC data of critical exchangers did not show or indicate any scaling. It was then suspected that physical fouling could have reduced the water flow through the condenser as some physical fouling had been observed earlier during the turn around. Detailed flow studies were conducted with ultrasonic flow meters and it was observed that the water flow had considerably reduced. Armed with this data, it was decided to back-flush the condenser. Back-flushing solved the problem and vacuum and process side temperatures were restored. This averted a shutdown of the plant.
PART IILATEST TRENDS:Let us now take a look at some of the latest trends in cooling water treatment. This can be looked at from;
• the chemical treatment side,
• monitoring and automated dosing systems and
• Operational aspects.
Chemical Treatment Programmes:As mentioned earlier the main problems associated with any cooling water system are corrosion, scaling, fouling and microbial growth. The most commonly adopted programme today includes zinc and orthophosphate as the main corrosion inhibitors along with organophosphonates and polymers for scale and deposit control. Microbial control is achieved with the continuous use of an oxidizing biocide, commonly chlorine, along with a biodispersant, which are specific surfactants, for the removal of slime and organic contaminants. Once these biofilm are prevented from adhering to the exchanger tubes and kept in suspension and circulation in the cooling water, the oxidizing biocide can attack and kill these organisms thereby preventing the build up of slime in the system. Periodically non-oxidizing biocides are used to supplement the action of chlorine and biodispersant.
What are the latest and the future trends in each of these areas are worth taking a look at.
Corrosion Inhibition:![Cathodic and an Anodic Corrosion Inhibitor Cathodic and an Anodic Corrosion Inhibitor]()
It is well established that a synergistic combination of a cathodic and an anodic corrosion inhibitor provides the best corrosion protection. However there are specific conditions or instances where these common inhibitors need to be further supplemented or replaced for better performance.
![Corrosion Inhibition Corrosion Inhibition]()
India has a wide spectrum of water quality ranging from lean low hardness and TDS water from one region to hard water with high TDS in other parts. Low hardness lean waters are more corrosive while higher hardness shifts the potential towards deposition. High TDS water with high concentrations of aggressive ions like chloride and sulfate can lead to higher corrosion potential. In this complex scenario supplementing common corrosion inhibitors like zinc and orthophosphate with newer molecules often become necessary.
Aromatic azoles have been used since long as a specific corrosion inhibitor for exchangers/condensers with metallurgy based on copper and copper alloys. Further work with these azoles has shown that a combination of azoles with organophosporus compounds and polymers have yielded good corrosion protection to ferrous metals also. Increasingly we shall find the incorporation of azoles in water treatment formulations for the corrosion protection of both ferrous and copper and copper alloy based metallurgy.
This finding has also made possible the operation of cooling water systems at alkaline pH (8-9) with all organic water treatment formulations. It becomes especially useful for units that do not want to use acid for the control of pH. At higher pH the corrosion potential is lower and the all organic composition provides adequate corrosion protection without the fear of deposition usually faced with inorganic salts used. Of course, microbial control in such situations need added attention.
Molybdates are another group of compounds used to enhance the corrosion protection particularly in situations where one encounters high temperatures, lean low hardness water and/or a system with deposits already present. Molybdate besides functioning as an anodic corrosion inhibitor can also from plugs on existing deposits making it impervious to aggressive ions like chloride and sulphate. Once these ions are prevented from reaching the metal under the deposits, their concentration cannot build up and further localized corrosion is arrested. The disadvantage of molybdate is its prohibitive price and that is the reason for its very selective use.
Today, using various combinations of corrosion inhibitor it is possible to obtain a corrosion rate of less than 1 mpy on regular basis.
Scale and Deposit ControlOperation of cooling water systems at high cycles of concentration is becoming the trend everywhere. This is essential considering the fact that water is increasingly becoming a very valuable and expensive resource.
![Scale deposit control Scale deposit control]()
Under such operating conditions the deposition tendency of the water increases manifold and good deposit control can be the difference between the success and failure of a treatment programme.
![Effect of scale on heat transfer Effect of scale on heat transfer]()
Organophosphonates and polymers used in combination have provided excellent deposit control. But there is a shift, more and more towards polymers in modern water treatment technology. Infact polymers form the most important and integral part of any successful non chromate cooling water treatment programme today. It has particularly assumed significance after the advent of non-chromate programmes where scale and fouling control is extremely critical while corrosion has still to be restricted to minimum limits. There are various polymers available, each with its strengths and weaknesses, and it is vital that the right polymers are selected in a treatment programme, taking into account the specific system needs and demands.
The most widely used polymers are low molecular weight (2000 to 20,000) and usually use acrylic acid as one of the monomers. Polymers can be;
• homo-polymers (using a single monomer),
• co-polymers (two monomers),
• ter-polymer (three monomers),
• Tetra-polymer (four monomers) and so on.
It is very important to know what the polymers specifically function as, in the selected programme. Polymers have to provide improved performance besides being cost effective and have to be function-specific along with improved performance.
Most polymers exhibit multifunctional properties like
• dispersion,
• threshold inhibition,
• crystal distortion and
• Chelation.
However each one excels in one particular aspect of control and should be evaluated as such. A polymer may exhibit excellent control of suspended matter while another may excel as an inhibitor of phosphate salts while providing multifunctional benefits of other control properties to varying degrees. Even today the best and field proven calcium carbonate inhibitor is an organophosphonate (Phosphono-Butane Tri-carboxylic acid) and selecting a polymer for the control of that function may not be a wise choice. Specific polymers are available for the control of individual scale forming salts, phosphate, metal ion foulants and suspended matter. Also, well-defined application tests are available to evaluate the superiority of, as well as determine the optimum feed levels of polymers for specific applications.
A well-designed cooling water treatment programme takes account of all these aspects and selects components depending on the actual system needs established after a thorough survey. This provides the basis of selection and includes application specific polymers along with other scale and corrosion inhibitors. Coupled with the total microbial control programme it forms the basis for the total cooling water treatment package.
New generation polymers have been designed for specific functions like;
• Phosphate inhibition
• Calcium carbonate inhibition
• Calcium sulfate inhibition
• Strontium sulfate inhibition
• Barium Sulfate inhibition
• Silica control
• Metal ion and their foulant control
• Suspended matter dispersion
Microbial Control:The primary mode of microbial control is with the use of an oxidizing biocide along with a bio-dispersant.
It is important to control the formation of biofilm very efficiently because they can cause the following problems;
• Reduction in plant performance by the growth of biofilm.
• Reduction in plant integrity due to microbial corrosion
• Reduction in plant safety due to the growth of legionella.
Biodispersants preferably should be none foaming or low foaming and latest biodispersant developed also incorporate ingredients that impart biostatic property to the product.
Biodispersants are essentially specific surfactants that target microbiological slime and biofilm and dislodge them from heat exchanger surfaces. Once brought into circulation, regular biocides (oxidizing or non-oxidizing) can then control these organisms by killing them.
Chlorine is one the oldest and cheapest oxidizing biocide used even today. The effort is to increase its effectiveness further by supplementing it with bromides or chlorine dioxide. This enhances the microbial control even in the presence of contaminants that otherwise use up chlorine and convert it to microbiologically useless chlorides. Also, it extends the effectiveness of control to alkaline pH where chlorine’s activity as a biocide is reduced.
Increasingly chlorine dioxide is being used as the product of choice.
Some of the major advantages of chlorine dioxide are:-
• ClO2 does not react with water, unlike other oxidants, nor does its chemical composition or biological activity change with shifts in the water’s pH.
• ClO2 in solution is actually a free radical which explains why it has an excellent biocidal capability. That is also why ClO2 disperses so quickly in water yet does not readily dissociate.
• ClO2 actually uses five available electrons to disrupt cellular metabolism of the cell to kill the organism and block enzymes necessary for metabolism. All other oxidizers are limited to only two available electrons
• ClO2 has 2/3 the oxidation potential of Cl2 but 2-1/2 times the oxidizing capacity of Cl2.
• ClO2, like Ozone, acts as a dissolved gas. 100% of the gas is a disinfectant. Only a portion of the other oxidizing solutions, are disinfectants
• Because ClO2 is a gas it penetrates many substances, including biofilm, much quicker and more effectively than other oxidizing or non-oxidizing biocides
• Unlike many other oxidizing or non-oxidizing biocides, ClO2penetrates the mucilaginous film of the biofilm without chemically altering its own composition
Ozone, another very clean oxidizing biocide, is being used in smaller applications. Its use and cost effectiveness is yet to be established in large cooling water systems. Similarly peroxygens have found limited use primarily due to cost effective reasons.
Many new molecules and various combinations are being tried by water treatment companies as effective non oxidizing biocides. The main concerns are safety, biodegrability of the actives and effectiveness against a broad spectrum of micro-organisms. The effectiveness in alkaline pH is another important concern as there is a shift towards operating cooling water systems in the alkaline pH range and earlier generation non oxidizing biocides lose their effectiveness in alkaline pH.
Non oxidizing biocides are expensive. The thrust today is to increasingly limit the use these compounds by reducing the frequency of addition and in some cases doing away with them altogether. In many cases, if the make up water is well treated and the system is not prone to leakages of organic contaminants, the use of a proper biodispersant along with chlorine and sometimes chlorine dioxide is sufficient for good microbial control.
Some of the future trends in microbial control that are being worked on are:
• Photodynamic disinfection
• Enzyme control of biofilm
• Interference with bacterial chemical messengers
• Bioelectric effect
• Ultrasound control of biofouling
• Magnetically-enhanced disinfection
• Surface-catalysed disinfection
Monitoring & Dosing:Attention, in recent times, is focused more on developing monitoring tools and feed systems. Concentration is on developing equipment and tools that simulate the operating conditions in the cooling water systems to provide meaningful data to ensure proactive management of the cooling water system and treatment programme.
Continuous monitoring and recording of corrosion rate and ORP (oxidation reduction potential) has almost become standard practice in many large cooling water systems. This is supplemented with data from various kinds of deposit and biofouling monitors to give a comprehensive picture of the treatment status. All these data can be made available on the DCS in the control room making monitoring even more stringent.
Certain important water parameters can also be continuously monitored with ion specific electrodes and specific test kits manufactured by specialist companies dedicated to their manufacture. These can also be provided by the water treatment companies as a part of their value added services. Once such data are available, automated feed systems can be devised to automatically run the feed pumps as per the parameters on continuous basis. Alternately one treatment chemical parameter can be analysed and all other continuously fed chemicals can be piggy backed on that parameter as per the ratio of the treatment chemicals fixed at the start of the programme. This is a less desired mode of operation as often the ratio needs to be changed depending on the changing system conditions which will require manual intervention.
Another interesting feed mode based on all these available data is to control the feed pumps with the help of a computer and a modem which can facilitate monitoring and operation of the feed pumps even from a remote location.
Besides vigilant monitoring these also assist in narrowing the band range of various chemicals that are maintained in the system bringing in an obvious and direct cost saving with respect to treatment chemical consumption.
Operational:This is an area where a good audit can be very handy. Of the various operational value additions like flow measurements, re-engineering, etc, the recycle of ETP water will become most important and critical in future operations.
As mentioned earlier water is increasingly becoming a very valuable and expensive resource. Its conservation and reuse will therefore be of prime importance in future operations of any plant. The reuse of ETP water is being considered and looked into by many plants all over India. In the years gone by the pollutants in the water were easily degradable and often biological degradation was sufficient. The nature of pollutants over the years has changed and with increasing amounts of non biodegradable contaminants being added the conventional treatment regime of wastewater may not be enough. The treatment philosophy will need to undergo a change depending on the nature of contaminants and biological degradation process will need to be supplemented or replaced with physico-chemical treatment systems : an area which involves most of the unit operations of chemical engineering, knowledge base for which is available in house and one need not look outwards.
ETP water, properly treated, are being used as partial make up in cooling water systems in plants where water shortage is acute and the cost excessive. There are also cases where the ETP water undergoes elaborate treatment with a final RO step and final treated water is used as boiler feed.
Ultimately, the plant and their selected water treatment company have to work as partners and pool in their combined specialist knowledge to create innovative solutions to suit the specific needs of individual installations. This, I believe, is the road to the future.











 X-rays were discovered in 1895 by Wilhelm Conrad Roentgen (1845-1923) who was a Professor at Wuerzburg University in Germany. Working with a cathode-ray tube in his laboratory, Roentgen observed a fluorescent glow of crystals on a table near his tube. The tube that Roentgen was working with consisted of a glass envelope (bulb) with positive and negative electrodes encapsulated in it. The air in the tube was evacuated, and when a high voltage was applied, the tube produced a fluorescent glow. Roentgen shielded the tube with heavy black paper, and discovered a green colored fluorescent light generated by a material located a few feet away from the tube.
X-rays were discovered in 1895 by Wilhelm Conrad Roentgen (1845-1923) who was a Professor at Wuerzburg University in Germany. Working with a cathode-ray tube in his laboratory, Roentgen observed a fluorescent glow of crystals on a table near his tube. The tube that Roentgen was working with consisted of a glass envelope (bulb) with positive and negative electrodes encapsulated in it. The air in the tube was evacuated, and when a high voltage was applied, the tube produced a fluorescent glow. Roentgen shielded the tube with heavy black paper, and discovered a green colored fluorescent light generated by a material located a few feet away from the tube.  Roentgen's discovery was a scientific bombshell, and was received with extraordinary interest by both scientist and laymen. Scientists everywhere could duplicate his experiment because the cathode tube was very well known during this period. Many scientists dropped other lines of research to pursue the mysterious rays. Newspapers and magazines of the day provided the public with numerous stories, some true, others fanciful, about the properties of the newly discovered rays.
Roentgen's discovery was a scientific bombshell, and was received with extraordinary interest by both scientist and laymen. Scientists everywhere could duplicate his experiment because the cathode tube was very well known during this period. Many scientists dropped other lines of research to pursue the mysterious rays. Newspapers and magazines of the day provided the public with numerous stories, some true, others fanciful, about the properties of the newly discovered rays.  Prior to 1912, X-rays were used little outside the realms of medicine and dentistry, though some X-ray pictures of metals were produced. The reason that X-rays were not used in industrial application before this date was because the X-ray tubes (the source of the X-rays) broke down under the voltages required to produce rays of satisfactory penetrating power for industrial purposes. However, that changed in 1913 when the high vacuum X-ray tubes designed by Coolidge became available. The high vacuum tubes were an intense and reliable X-ray source, operating at energies up to 100,000 volts.
Prior to 1912, X-rays were used little outside the realms of medicine and dentistry, though some X-ray pictures of metals were produced. The reason that X-rays were not used in industrial application before this date was because the X-ray tubes (the source of the X-rays) broke down under the voltages required to produce rays of satisfactory penetrating power for industrial purposes. However, that changed in 1913 when the high vacuum X-ray tubes designed by Coolidge became available. The high vacuum tubes were an intense and reliable X-ray source, operating at energies up to 100,000 volts.
 The radiation dose is directly proportional to the time spent in the radiation. Therefore, a person should not stay near a source of radiation any longer than necessary. If a survey meter reads 4 mR/h at a particular location, a total dose of 4mr will be received if a person remains at that location for one hour. In a two hour span of time, a dose of 8 mR would be received. The following equation can be used to make a simple calculation to determine the dose that will be or has been received in a radiation area.
The radiation dose is directly proportional to the time spent in the radiation. Therefore, a person should not stay near a source of radiation any longer than necessary. If a survey meter reads 4 mR/h at a particular location, a total dose of 4mr will be received if a person remains at that location for one hour. In a two hour span of time, a dose of 8 mR would be received. The following equation can be used to make a simple calculation to determine the dose that will be or has been received in a radiation area.





 Although any organ or tissue may develop a tumor after overexposure to radiation, certain organs and tissues seem to be more sensitive in this respect than others. Radiation-induced cancer is observed most frequently in the hemopoietic system, in the thyroid, in the bone, and in the skin. In all these cases, the tumor induction time in man is relatively long - on the order of 5 to 20 years after exposure.
Although any organ or tissue may develop a tumor after overexposure to radiation, certain organs and tissues seem to be more sensitive in this respect than others. Radiation-induced cancer is observed most frequently in the hemopoietic system, in the thyroid, in the bone, and in the skin. In all these cases, the tumor induction time in man is relatively long - on the order of 5 to 20 years after exposure. Leukemia is a cancer of the early blood-forming cells. Usually, the leukemia is a cancer of the white blood cells, but leukemia can involve other blood cell types as well. Leukemia starts in the bone marrow and then spreads to the blood. From there it can go to the lymph nodes, spleen, liver, central nervous system (the brain and spinal cord), testes (testicles), or other organs. Leukemia is among the most likely forms of malignancy resulting from overexposure to total body radiation. Chronic lymphocytic leukemia does not appear to be related to radiation exposure.
Leukemia is a cancer of the early blood-forming cells. Usually, the leukemia is a cancer of the white blood cells, but leukemia can involve other blood cell types as well. Leukemia starts in the bone marrow and then spreads to the blood. From there it can go to the lymph nodes, spleen, liver, central nervous system (the brain and spinal cord), testes (testicles), or other organs. Leukemia is among the most likely forms of malignancy resulting from overexposure to total body radiation. Chronic lymphocytic leukemia does not appear to be related to radiation exposure. The genetic information can be altered by many different chemical and physical agents called mutagens, which disrupt the sequence of bases in a DNA molecule. If this information content of a somatic cell is scrambled, then its descendants may show some sort of an abnormality. If the information that is jumbled is in a germ cell that subsequently is fertilized, then the new individual may carry a genetic defect, or a mutation. Such a mutation is often called a point mutation, since it results from damage to one point on a gene. Most geneticists believe that the majority of such mutations in man are undesirable or harmful.
The genetic information can be altered by many different chemical and physical agents called mutagens, which disrupt the sequence of bases in a DNA molecule. If this information content of a somatic cell is scrambled, then its descendants may show some sort of an abnormality. If the information that is jumbled is in a germ cell that subsequently is fertilized, then the new individual may carry a genetic defect, or a mutation. Such a mutation is often called a point mutation, since it results from damage to one point on a gene. Most geneticists believe that the majority of such mutations in man are undesirable or harmful. A cataract is a clouding of the normally clear lens of the eye. A much higher incidence of cataracts was reported among physicists in cyclotron laboratories whose eyes had been exposed intermittently for long periods of time to relatively low radiation fields, as well as among atomic bomb survivors whose eyes had been exposed to a single high radiation dose. This shows that both chronic and acute overexposure of the eyes can lead to cataracts. Radiation may injure the cornea, conjunctiva, iris, and the lens of the eye. In the case of the lens, the principal site of damage is the proliferating cells of the anterior epithelium. This results in abnormal lens fibers, which eventually disintegrate to form an opaque area, or cataract, that prevents light from reaching the retina.
A cataract is a clouding of the normally clear lens of the eye. A much higher incidence of cataracts was reported among physicists in cyclotron laboratories whose eyes had been exposed intermittently for long periods of time to relatively low radiation fields, as well as among atomic bomb survivors whose eyes had been exposed to a single high radiation dose. This shows that both chronic and acute overexposure of the eyes can lead to cataracts. Radiation may injure the cornea, conjunctiva, iris, and the lens of the eye. In the case of the lens, the principal site of damage is the proliferating cells of the anterior epithelium. This results in abnormal lens fibers, which eventually disintegrate to form an opaque area, or cataract, that prevents light from reaching the retina.