Ammonia Production
In 1909 Fritz Haber established the conditions under which nitrogen, N
2(g), and hydrogen, H
2(g), would combine using
- medium temperature (~500oC)
- very high pressure (~250 atmospheres, ~351kPa)
- a catalyst (a porous iron catalyst prepared by reducing magnetite, Fe3O4).
Osmium is a much better catalyst for the reaction but is very expensive.
This process produces an ammonia, NH
3(g), yield of approximately 10-20%.
The Haber synthesis was developed into an industrial process by Carl Bosch.
The reaction between nitrogen gas and hydrogen gas to produce ammonia gas is
exothermic, releasing 92.4kJ/mol of energy at 298K (25
oC).
N2(g)
nitrogen | + | 3H2(g)
hydrogen | heat, pressure, catalyst
![]() | 2NH3(g)
ammonia | ![]() H = -92.4 kJ mol-1 H = -92.4 kJ mol-1 |
By
Le Chetalier's Principle:- increasing the pressure causes the equilibrium position to move to the right resulting in a higher yeild of ammonia since there are more gas molecules on the left hand side of the equation (4 in total) than there are on the right hand side of the equation (2). Increasing the pressure means the system adjusts to reduce the effect of the change, that is, to reduce the pressure by having fewer gas molecules.
- decreasing the temperature causes the equilibrium position to move to the right resulting in a higher yield of ammonia since the reaction is exothermic (releases heat). Reducing the temperature means the system will adjust to minimise the effect of the change, that is, it will produce more heat since energy is a product of the reaction, and will therefore produce more ammonia gas as well
However, the rate of the reaction at lower temperatures is extremely slow, so a higher temperature must be used to speed up the reaction which results in a lower yield of ammonia.
| The equilibrium expression for this reaction is: | | As the temperature increases, the equilibrium constant decreases as the yield of ammonia decreases. | | Temperature (oC) | Keq |
|---|
| 25 | 6.4 x 102 | | 200 | 4.4 x 10-1 | | 300 | 4.3 x 10-3 | | 400 | 1.6 x 10-4 | | 500 | 1.5 x 10-5 |
|
Rate considerations:
- A catalyst such as an iron catalyst is used to speed up the reaction by lowering the activation energy so that the N2 bonds and H2 bonds can be more readily broken.
- Increased temperature means more reactant molecules have sufficient energy to overcome the energy barrier to reacting (activation energy) so the reaction is faster at higher temperatures (but the yield of ammonia is lower as discussed above).
A temperature range of 400-500oC is a compromise designed to achieve an acceptable yield of ammonia (10-20%) within an acceptable time period.
At 200oC and pressures above 750atm there is an almost 100% conversion of reactants to the ammonia product.
Since there are difficulties associated with containing larger amounts of materials at this high pressure, lower pressures of around 200 atm are used industrially.
By using a pressure of around 200atm and a temperature of about 500oC, the yield of ammonia is 10-20%, while costs and safety concerns in the biulding and during operation of the plant are minimised | ![]() |
During industrial production of ammonia, the reaction never reaches equilibrium as the gas mixture leaving the reactor is cooled to liquefy and remove the ammonia. The remaining mixture of reactant gases are recycled through the reactor. The heat released by the reaction is removed and used to heat the incoming gas mixture.
Uses of Ammonia
| Industry | Use |
|---|
| Fertilser | production of:
- ammonium sulfate, (NH4)2SO4
- ammonium phosphate, (NH4)3PO4
- ammonium nitrate, NH4NO3
- urea, (NH2)2CO,also used in the production of barbiturates (sedatives), is made by the reaction of ammonia with carbon dioxide
CO2
carbon dioxide | | + | 2NH3
ammonia | ![]() | H2NCOONH4
ammonium carbonate | heat, pressure
![]() | (NH2)2CO
urea |
|
|---|
| Chemicals | synthesis of:
- nitric acid, HNO3, which is used in making explosives such as TNT (2,4,6-trinitrotoluene), nitroglycerine which is also used as a vasodilator (a substance that dilates blood vessels) and PETN (pentaerythritol nitrate).
- sodium hydrogen carbonate (sodium bicarbonate), NaHCO3
- sodium carbonate, Na2CO3
- hydrogen cyanide (hydrocyanic acid), HCN
- hydrazine, N2H4 (used in rocket propulsion systems)
|
|---|
| Explosives | ammonium nitrate, NH4NO3 |
|---|
| Fibres & Plastics | nylon, -[(CH2)4-CO-NH-(CH2)6-NH-CO]-,and other polyamides |
|---|
| Refrigeration | used for making ice, large scale refrigeration plants, air-conditioning units in buildings and plants |
|---|
| Pharmaceuticals | used in the manufacture of drugs such as sulfonamide which inhibit the growth and multiplication of bacteria that require p-aminobenzoic acid (PABA) for the biosynthesis of folic acids, anti-malarials and vitamins such as the B vitamins nicotinamide (niacinamide) and thiamine. |
|---|
| Pulp & Paper | ammonium hydrogen sulfite, NH4HSO3, enables some hardwoods to be used |
|---|
| Mining & Metallurgy | used in nitriding (bright annealing) steel,
used in zinc and nickel extraction |
|---|
| Cleaning | ammonia in solution is used as a cleaning agent such as in 'cloudy ammonia' |
|---|
A Brief History
At the beginning of the 20
th century there was a shortage of naturally occurring, nitrogen-rich fertilisers, such as Chile saltpetre, which prompted the German Chemist Fritz Haber, and others, to look for ways of combining the nitrogen in the air with hydrogen to form ammonia, which is a convenient starting point in the manufacture of fertilisers.This process was also of interest to the German chemical industry as Germany was preparing for World War I and nitrogen compounds were needed for explosives.
The hydrogen for the ammonia synthesis was made by the water-gas process (a Carl Bosch invention) which involves blowing steam through a bed of red hot coke resulting in the separation of hydrogen from oxygen. The nitrogen was obtained by distillation of liquid air, then by cooling and compressing air.
These days, the hydrogen is produced by reforming light petroleum fractions or natural gas (methane, CH
4) by adding steam:
| CH4(g) + H2O(g) | Ni catalyst
---------->
700oC | CO(g) + 3H2(g) |
Enough steam is used to react with about 45% of the methane (CH
4), the rest of the methane is reacted with air:
| 2CH4(g) + | O2(g) + 4N2(g)
(air) | Ni catalyst
---------> | 2CO(g) + 4H2(g) + 4N2(g) |
All the carbon monoxide (CO) in the mixture is oxidised to CO
2 using steam and an iron oxide catalyst:
| CO(g) + H2O(g) | iron oxide catalyst
------------------> | H2(g) + CO2(g) |
The carbon dioxide
(CO2)is removed using a suitable base so that only the nitrogen gas
(N2)and hydrogen gas
(H2)remain and are used in the production of
ammonia (NH3).
In ammonia production the hydrogen and nitrogen are mixed together in a ratio of 3:1 by volume and compressed to around 200 times atmospheric pressure.
Production
Feedstock:The original feedstock for the old 1,000-ton-per day Allied Chemical ammonia plant at Hopewell, VA, USA, was water gas (i.e., illuminating gas), the same type of gas that was manufactured for heating, lighting and cooking in cities before natural gas became available. It was made from coke and steam. Allied/Hopewell made enough such gas per day to have supplied a city the size of New York
Feedstocks may range from, ethane through petroleum naphtha to petroleum gas oils. The basic processing scheme used for such feedstocks is the same as for a natural gas feedstock, however ammonia eventually is made by mixing hydrogen with nitrogen. It requires a catalytic conversion reactor followed by cooling the hot reactor effluent gas: first by generating steam with the hot effluent gas and then by using mechanical refrigeration to separate out the liquified anhydrous ammonia. It also requires compressing the catalytic reactor feed gases to a range of 100 to 250 bar, depending on the reactor process being used.
Here is the ammonia process with Gas feedstock which is a guideline to the original and most used process.
2. DESCRIPTION OF PRODUCTION PROCESSES2.1 Production Processes in Operation in EuropeTwo main types of production process for ammonia synthesis gas are currently in operation in Europe:-
- Steam reforming of natural gas or other light hydrocarbons (Natural Gas Liquids,
Liquefied Petroleum Gas, Naphtha)
- Partial oxidation of heavy fuel oil or vacuum residue
In at least one partial oxidation unit, natural gas is used as feedstock. Coal gasification and water electrolysis are no longer in use in the European ammonia industry.
The ammonia synthesis process is principally independent of the type of synthesis gas pro-duction process, but synthesis gas quality influences the loop design and operating conditions.
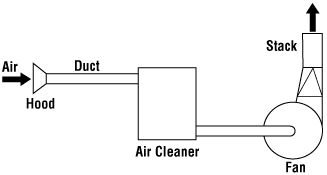
A block diagram of the conventional steam reforming process is shown in Figure 1. (In some cases, a separate auxiliary boiler is required). About 85% of world ammonia production is based on steam reforming concepts [3]. A process description is given in 2.2.1.
For heavier feedstocks than naphtha, partial oxidation with oxygen is used in the synthesis gas production. A block diagram of a typical partial oxidation process is shown in Figure 2, and a process description is given in 2.2.5.
2.2 BAT Production ProcessesNo single process can be identified as BAT for the production of ammonia. In this chapter the characteristics of BAT processes based on available feedstocks are described. Other BAT processes may also exist.
Natural gas reforming with steam and air is the simplest and most efficient way of ammonia synthesis gas production. Comparing natural gas reforming, heavy oil and coal gasification gives the following approximate relative consumption figures, based on modern technological standards for each route, at European economic conditions:-
| Natural Gas | Heavy Oil | Coal |
| Energy consumption | 1.0 | 1.3 | 1.7 |
| Investment cost | 1.0 | 1.4 | 2.4 |
| Production cost | 1.0 | 1.2 | 1.7 |
Based on the known resources of fossil raw materials, it is likely that natural gas will domi-nate as the feedstock for ammonia for the next 50 years at least. In the very long term, 50-200 years, one might expect coal to take over, based on world reserves and consumption rate. Heavy oil may be attractive under special environmental concerns, when natural gas is not available and the partial oxidation process could solve a waste problem (heavy residues, plastics recycle).
For the present time and the near future, the steam/air reforming concepts based on natural gas and other light hydrocarbons are considered to be the dominating group of BAT production processes. The BAT reforming processes can be divided into the following types:-
- Conventional steam reforming with a fired primary reformer and stoichiometric air secondary reforming (stoichiometric H/N-ratio)
- Steam reforming with mild conditions in a fired primary reformer and excess air sec-ondary reforming (under-stoichiometric H/N-ratio)
- Heat exchange autothermal reforming, with a process gas heated steam reformer (heat exchange reformer) and a separate secondary reformer, or in a combined autothermal reformer, using excess or enriched air (under-stoichiometric or stoichio-metric H/N-ratio)
For heavy feedstocks, partial oxidation is considered as the BAT production process.
All three reforming versions, and partial oxidation of heavy residues, are operated in Europe today.
The following description concentrates on presenting the conventional steam reforming process and only deviations and additions will be described for the other BAT processes.
2.2.1 Conventional steam reforming2.2.1.1 Overall conversionThe theoretical process conversions, based on methane feedstock, are given in the following approximate formulae:-
0.88CH4 + 1.26Air + 1.24H2O -----> 0.88CO2 + N2 + 3H2
N2 + 3H2 -----> 2NH3The synthesis gas production and purification normally take place at 25-35bar pressure. The ammonia synthesis pressure is usually in the range 100-250bar. For more detailed process flow sheets refer to Ullmann [3].
2.2.1.2 Feedstock desulphurisationMost of the catalysts used in the process are sensitive to sulphur and sulphur compounds. The feedstock normally contains up to 5mg S.Nm
-3 as sulphur compounds. The feed-gas is pre-heated to 350-400 °C, usually in the primary reformer convection section, and then treated in a desulphurisation vessel, where the sulphur compounds are hydrogenated to H
2S, typically using a cobalt molybdenum catalyst, and then adsorbed on
pelletised zinc oxide:-
R-SH + H2 -----> H2 S + RH
H2 S + ZnO -----> ZnS + H2OIn this way, the sulphur is removed to less than 0.1ppm S in the gas feed. The zinc sulphide remains in the adsorption bed. The hydrogen for the reaction is usually recycled from the synthesis section.
2.2.1.3 Primary reformingThe gas from the desulphuriser is mixed with process steam, usually coming from an extraction turbine, and the steam/gas mixture is then heated further to 500-600 °C in the convection section before entering the primary reformer. In some new or revamped plants the preheated steam/gas mixture is passed through an adiabatic pre-reformer and reheated in the convection section, before entering the primary reformer. (Special pre-reformer catalysts are offered by several suppliers). Also, in some plants, part of the process steam is supplied by feed-gas saturation.
The amount of process steam is given by the process steam to carbon molar ratio (S/C-ratio), which should be around 3.0 for the BAT reforming processes. The optimum ratio depends on several factors, such as feedstock quality, purge gas recovery, primary reformer capacity, shift operation, and the plant steam balance. In new plants the optimum S/C-ratio may be lower than 3.0.
The primary reformer consists of a large number of high-nickel chromium alloy tubes filled with nickel-containing reforming catalyst. The overall reaction is highly endothermic and additional heat is required to raise the temperature to 780-830 °C at the reformer outlet.
The composition of the gas leaving the primary reformer is given by close approach to the following chemical equilibria:-
CH4 + H20 <---> CO + 3H2 ![wpe19.jpg (679 bytes)]() H0298 = 206 kJ.mol-1
H0298 = 206 kJ.mol-1C0 + H
20 <---> CO
2 + H
2 ![wpe19.jpg (679 bytes)]()
H
0298 = -41 kJ.mol
-1The heat for the primary reforming process is supplied by burning natural gas or other gaseous fuel, in the burners of a radiant box containing the tubes.
The flue-gas leaving the radiant box has temperatures in excess of 900 °C, after supplying the necessary high level heat to the reforming process. Thus only about 50-60% of the fuel’s heat value is directly used in the process itself. The heat content (waste heat) of the flue-gas is used in the reformer convection section, for various process and steam system duties. The fuel energy requirement in the conventional reforming process is 40-50% of the process feed-gas energy.
The flue-gas leaving the convection section at 100-200 °C is one of the main sources of emissions from the plant. These emissions are mainly
CO2,
NOx, with small amounts of
SO2and
CO.
2.2.1.4 Secondary reformingOnly 30-40% of the hydrocarbon feed is reformed in the primary reformer because of the chemical equilibria at the actual operating conditions. The temperature must be raised to increase the conversion. This is done in the secondary reformer by internal combustion of part of the gas with the process air, which also provides the nitrogen for the final synthesis gas. In the conventional reforming process the degree of primary reforming is adjusted so that the air supplied to the secondary reformer meets both the heat balance and the stoichiometric synthesis gas requirement.
The process air is compressed to the reforming pressure and heated further in the primary reformer convection section to around 600 °C. The process gas is mixed with the air in a burner and then passed over a nickel-containing secondary reformer catalyst. The reformer outlet temperature is around 1,000 °C, and up to 99% of the hydrocarbon feed (to the primary reformer) is converted, giving a residual methane content of 0.2-0.3% (dry gas base) in the process gas leaving the secondary reformer.
The process gas is cooled to 350-400 °C in a waste heat steam boiler or boiler/superheater downstream from the secondary reformer.
2.2.1.5 Shift conversionThe process gas from the secondary reformer contains 12-15%
CO(dry gas base) and most of the
COis converted in the shift section according to the reaction:-
CO + H2O <---> CO2 + H2 ![wpe19.jpg (679 bytes)]() HO298 = -41 kJ.mol-1
HO298 = -41 kJ.mol-1In the High Temperature Shift (HTS) conversion, the gas is passed through a bed of iron oxide/chromium oxide catalyst at around 400 °C, where the
COcontent is reduced to about 3% (dry gas base), limited by the shift equilibrium at the actual operating temperature. There is a tendency to use copper containing catalyst for increased conversion. The gas from the HTS is cooled and passed through the Low Temperature Shift (LTS) converter.
This LTS converter is filled with a copper oxide/zinc oxide-based catalyst and operates at about 200-220 °C. The residual
COcontent in the converted gas is about 0.2-0.4% (dry gas base). A low residual CO content is important for the efficiency of the process.
2.2.1.6 CO2 removalThe process gas from the low temperature shift converter contains mainly
H2,
N2,
CO2and the excess process steam. The gas is cooled and most of the excess steam is condensed before it enters the
CO2removal system. This condensate normally contains 1,500-2,000ppm of
ammonia800-1,200ppm of
methanol. Minor amounts of
amines,
formic acidand
acetic acidcould be present in the condensate. All these components should be stripped from the condensate and/or recycled in BAT processes. The heat released during cooling/condensation is used for:-
-The regeneration of the
CO2scrubbing solution
- Driving an absorption refrigeration unit
- Boiler feedwater preheat
The amount of heat released depends on the process steam to carbon ratio. If all this low-level heat is used for
CO2removal or absorption refrigeration, high-level heat has to be used for the feedwater system. An energy-efficient process should therefore have a
CO2removal system with a low heat demand.
The
CO2is removed in a chemical or a physical absorption process. The solvents used in chemical absorption processes are mainly aqueous amine solutions (
Mono Ethanolamine (MEA),
Activated MethylDiEthanolamine (aMDEA)or hot
potassium carbonatesolutions. Physical solvents are glycol dimethylethers (Selexol), propylene carbonate and others. The MEA process has a high regeneration energy consumption and is not regarded as a BAT process.
For new ammonia plants the following CO 2 removal processes are currently regarded as BAT:-
- aMDEA standard 2-stage process, or similar
- Benfield process (HiPure, LoHeat), or similar
- Selexol or similar physical absorption processes
Concepts such as Pressure Swing Adsorption (PSA) should also be regarded as BAT in some new plants but in such cases
CO2removal is not the only function of the PSA unit.
The typical range of heat consumption in the modern chemical absorption process is 30-60MJ.kmol
-1CO2. The physical absorption processes may be designed for zero heat consumption, but for comparison with the chemical processes, the mechanical energy requirements have also to be considered.
Residual
CO2contents are usually in the range 100-1,000ppmv, dependent on the type and design of the removal unit. Contents down to about 50ppmv are achievable.
2.2.1.7 MethanationThe small amounts of CO and
CO2, remaining in the synthesis gas, are poisonous for the ammonia synthesis catalyst and must be removed by conversion to CH
4 in the methanator:-
CO + 3H2 -----> CH4 + H2OCO2+ 4H
2 -----> CH
4 + 2H
2O
The reactions take place at around 300 °C in a reactor filled with a nickel containing cata-lyst. Methane is an inert gas in the synthesis reaction, but the water must be removed before entering the converter. This is done firstly by cooling and condensation downstream of the methanator and finally by condensation/absorption in the product ammonia in the loop or in a make-up gas drying unit.
2.2.1.8 Synthesis gas compression and ammonia synthesisModern ammonia plants use centrifugal compressors for synthesis gas compression, usually driven by steam turbines, with the steam being produced in the ammonia plant. The refrigeration compressor, needed for condensation of product ammonia, is also usually driven by a steam turbine.
The synthesis of ammonia takes place on an iron catalyst at pressures usually in the range 100-250bar and temperatures in the range 350-550 °C:-
N2 + 3H2 <---> 2NH3 ![wpe19.jpg (679 bytes)]() H0298 = -46 kJ.mol -1 NH 3
H0298 = -46 kJ.mol -1 NH 3Only 20-30% is reacted per pass in the converter due to the unfavourable equilibrium conditions. The ammonia that is formed is separated from the recycle gas by cooling/condensa-tion, and the reacted gas is substituted by the fresh make-up synthesis gas, thus maintaining the loop pressure. In addition, extensive heat exchange is required due to the exothermic reaction and the large temperature range in the loop. A newly developed ammonia synthesis catalyst containing ruthenium on a graphite support has a much higher activity per unit of volume and has the potential to increase conversion and lower operating pressures.
Synthesis loop arrangements differ with respect to the points in the loop at which the make-up gas is delivered and the ammonia and purge gas are taken out. The best arrangement is to add the make-up gas after ammonia condensation and ahead of the converter. The loop purge should be taken out after ammonia separation and before make-up gas addition. This configuration is dependent on the make-up gas being treated in a drying step before entering the loop. A make-up gas containing traces of water or carbon dioxide must be added before ammonia condensation, with negative effects both to ammonia condensation and energy.
Conventional reforming with methanation as the final purification step, produces a synthesis gas containing inerts (methane and argon) in quantities that do not dissolve in the condensed ammonia. The major part of these inerts is removed by taking out a purge stream from the loop. The size of this purge stream controls the level of inerts in the loop to about 10-15%. The purge gas is scrubbed with water to remove ammonia before being used as fuel or before being sent for hydrogen recovery.
Ammonia condensation is far from complete if cooling is with water or air and is usually not satisfactory (dependent on loop pressure and cooling medium temperature). Vapourising ammonia is used as a refrigerant in most ammonia plants, to achieve sufficiently low ammonia concentrations in the gas recycled to the converter. The ammonia vapours are liquified after recompression in the refrigeration compressor.
2.2.1.9 Steam and power systemSteam reforming ammonia plants have high-level surplus heat available for steam production in the reforming, shift conversion, and synthesis sections, and in the convection section of the primary reformer. Most of this waste heat is used for high pressure steam production for use in turbines for driving the main compressors and pumps and as process steam extracted from the turbine system.
A modern steam reforming ammonia plant can be made energetically self-sufficient if nec-essary, but usually a small steam export and electricity import are preferred.
2.2.2 Steam reforming with excess air secondary reforming2.2.2.1 Process flowsheetSome processes are designed for reduced primary reforming by moving some of the duty to the secondary reformer because of the marginal low efficiency of the primary reformer. A brief description is given of features diverging from the conventional concept in the fol-lowing paragraphs. These features are:
--Decreased firing in the primary reformer
- Increased process air flow to the secondary reforming
- Cryogenic final purification after methanation
- Lower inert level of the make-up syngas
2.2.2.2 Decreased firing in the primary reformerDecreased heat supply in the primary reformer means that the process outlet temperature is lowered (to about 700 °C), the firing efficiency increases, and the size and cost of the primary reformer are reduced. The milder operating conditions prolong catalyst, catalyst tube and outlet header service lives. The extent of reforming is reduced according to the lower heat supply and lower temperature. Generally, a slight decrease in steam to carbon ratio is acceptable, compared to the conventional concept.
2.2.2.3 Increased process air supply to the secondary reformerDecreased heat supply in the primary reformer means that increased internal firing is necessary to achieve approximately the same degree of total reforming. A somewhat higher methane slip, and thus a lower secondary reformer outlet temperature is acceptable and preferable in this type of process, as methane is removed in the final purification step.
The process air requirement is about 50% higher than in the conventional process. This means increased compression capacity and energy. The process air compressor is usually driven by a gas turbine with the exhaust gas from the turbine being used as combustion air in the primary reformer. Some excess steam is available for export when using a gas turbine.
2.2.2.4 Cryogenic final purificationIn the cryogenic purifier all the methane and the excess nitrogen are removed from the synthesis gas as well as a part of the argon. The cooling is produced by depressurisation and no external supply is needed. The purified syngas is then practically free of all impurities, except for a small amount of argon. The cryogenic unit also receives the purge from the synthesis section and delivers an off-gas for fuel.
2.2.2.5 Lower syngas inert levelThe removal of essentially all impurities from the make-up synthesis gas is a significant improvement, compared to the conventional purification by methanation only. Higher conversion per pass and reduced purge flow, together result in a more efficient process.
2.2.3 Heat exchange autothermal reformingFrom a thermodynamic point of view it is wasteful to use the high-level heat of the secondary reformer outlet gas and the primary reformer flue-gas, both at temperatures around 1,000 °C, simply to raise steam. Recent developments are to recycle this heat to the process itself, by using the heat content of the secondary reformed gas in a newly-developed primary reformer (gas heated reformer, heat exchange reformer), thus eliminating the fired furnace. Surplus air or oxygen-enriched air is required in the secondary reformer to meet the heat balance in this autothermal concept.
Emissions to the atmosphere are reduced significantly by eliminating the flue-gas from the primary reformer. NO
x emissions may be reduced by 50% or more, depending on the extent of auxiliary combustion in the plant, compared to conventional steam reforming.
Two processes of this kind are in operation, and some others are at the pilot stage. All these processes are considered as BAT production processes. Recently it has been reported that capacities in the range of 1,800t.d
-1 can be built.
2.2.4 BAT reforming processes for new plantsThe modern versions of the conventional steam reforming and excess air reforming processes will still be used for new plants for many years to come. Developments are expected to go in the following directions:-
- Lowering the steam to carbon ratio
- Shifting duty from primary to secondary reformer
- Improved final purification
- Improved synthesis loop efficiency
- Improved power energy system
- Low NO
x burners
- Non iron based ammonia synthesis catalyst
The new autothermal concepts are expected to be developed further, and will continue the developments outlined above.
2.2.5 Partial oxidation of heavy oils2.2.5.1 Process descriptionThe partial oxidation process is used for the gasification of heavy feedstocks such as residual oils and coal. Extremely viscous hydrocarbons and plastic wastes may also be used as fractions of the feed. The partial oxidation process offers an alternative for future utilisation of such wastes.
An air separation unit is required for the production of oxygen for the partial oxidation step. The nitrogen is added in the liquid nitrogen wash to remove impurities from the synthesis gas and to get the required hydrogen/nitrogen ratio in the synthesis gas.
The partial oxidation gasification is a non-catalytic process taking place at high pressure (>50bar) and temperatures around 1,400 °C. Some steam is added for temperature modera-tion. The simplified reaction pattern is:-
-CHn - + 0.5O2 -----> CO + n/2H2Carbon dioxide, methane and some soot are formed in addition. The sulphur compounds in the feed are converted to hydrogen sulphide. Mineral compounds in the feed are transformed into specific ashes. The process gas is freed from solids by water scrubbing after waste heat recovery and the soot is recycled to the feed. The ash compounds are drained with the process condensate and/or together with a part of the soot. In at least two units in Europe, the soot is separated from soot water in a mainstream filtration stage, to avoid ash build-up in the gasification cycle downstream units. The heavy metals, such as V, Ni and Fe are recovered. The hydrogen sulphide in the process gas is separated in a selective absorption step and reprocessed to elementary sulphur in a Claus unit.
The shift conversion usually has two high temperature shift catalyst beds with intermediate cooling. Steam for the shift conversion is supplied partially by a cooler-saturator system and partially by steam injection.
CO2is removed by using an absorption agent which might be the same as that in the sulphur removal step. Residual traces of absorption agent and
CO2are then removed from the process gas, before final purification by a liquid nitrogen wash. In this unit practically all the impurities are removed and nitrogen is added to give the stoichiometric hydrogen to nitrogen ratio.
The ammonia synthesis is quite similar to that used in steam reforming plants, but simpler and more efficient, due to the high purity of synthesis gas from liquid nitrogen wash units and the synthesis loop not requiring a purge.
2.2.5.2 Steam and power systemAuxiliary boilers are required if the compressors are steam-driven. The flue-gas from these power plants is the main source of emissions which are mainly
SO2,
NOx, and
CO2. The site emissions are very low if the compressors are driven by imported electric power.
2.2.5.3 Future improvementsNo major improvements are to be expected concerning process efficiency and plant investment costs. However, partial oxidation will continue to be interesting in the future, due to its feedstock flexibility. The separation and disposal of the soot and especially the ashes are necessary to adapt to deteriorating residue qualities or alternative raw material sources.
General failures:failure of the air coil installed in the primary reformer
absorber and stripper vents fires
Corrosion:It all depends on the system. If you install a stainless steel valve in direct contact with cast iron piping, you may induce galvanic corrosion. Check OSHA's web site for ammonia. They have a picture of completely corroded SS piping.
Stainless may still be the best. Just fully address all mechanical integrity questions. Set up a periodic assessment of fitness for continued service.
An ultrsasonic device can measure inside corrosion under intact outside coating. It may be a good idea to buy the equipment ($500-1,000) and continuously check your entire system.
Pump ware:
Not being sure as to the exact problem with the o-rings, is it swelling, shrinking or explosive decompression..little chunks missing. Ammonia can be a difficult product to seal in pumps. We consulted with Parker O-Ring and started using a Neoprene C-873-70 for rotary vane pump mechanical seals.
Most of those installations problems subsided once we used it. I would contact the Parker Rep. Our office in Houston did and got the help we needed.
I had the same problems in a Viking pump, used as a low temperature transfer pump. I was told that these pumps were noted for blowing their o-rings. I bit the bullet and installed a Cornell transfer pump and I haven't had any problems since. This may be the solution to your problem. It sure did fix mine.
Contacts for processing:There are several companies you can contact to discuss your questions. First is EEC in Baltimore Md. Their web site and contact info can be found at www.eec1.com. Might talk to Herbert Spencer at EEC. Next is Wahlco in Santa Ana, California and they are at www.wahlco.com. Talk to Barry Southam or Mike Fujita. Another urea system is offered by Fuel Tech and they can also be found on the web. Talk to William Sun after finding their contact information on the web.
Another urea system is offered by Chemithon in Seattle Wa. Talk to Ken Reimers at 206-937-1187 or send an e-mail to: kreimers@chemithon.com.
These companies are the leaders in urea use for power plant DeNOx sytems that require an ammonia source from urea, that I am aware of at this time.
Economic considerations:The total ammonia production capacity in the United States is 19,500,000 tons per year. As of January 1, 2001, the amount of ammonia capacity operating was 12,000,000 tons per year. It is expected that the 7,500,000 tons per year of unused capacity will not be used until the price of natural gas falls below $6 per 1,000,000 BTUs
Waste retrofitting, and EnvirnomentThe production of ammonia can be divided into six main steps:
- Synthesis Gas Preparation: which includes desulfurization; steam reforming, coal gasification, oil oxidation, electrolysis; and shift conversion.
- Syn Gas Purification: which includes Carbon dioxide removal, methanation, etc.
- Syn Gas Compression.
- Ammonia Synthesis: reaction of hydrogen and nitrogen to form ammonia.
- Ammonia Separation and Recovery.
- Recycle: recycle of un-recovered ammonia and the syn gas back to the synthesis compressor.
The complex reaction involved in the production ammonia leads to the generation of a number of wastes. These wastes are mostly in gaseous form. During the recent years the fertilizer industry has implemented many waste reduction methods and techniques. As a result of which today the waste release and generation is much lower than what it was a few years ago. According to the Fertilizer Institute (TFI 2001), since 1987, the waste emissions in the U.S alone have decreased by approximately 75% in the nitrogen fertilizer industry [15].
Control & Conservation Techniques:
The following methods and techniques can be used for controlling emissions from ammonia production plants:
Using Natural Gas as the raw material:The use of natural gas as the raw material (feed stock) is the best way to decrease the waste emissions from an ammonia plant. The natural gas has high hydrogen content and a higher heating value than most of the hydrocarbons. Processes based on heavy hydrocarbons such as oils and coal, generate waste many time greater than natural gas based plants.
Excess Air Reforming:The IFA recommends the use of excess air reforming for improving the waste conservation and control of the ammonia plants [2]. The recommendation is based upon the fact that primary reformers have low efficiencies. By reducing the share of primary reformer in the reforming reaction, the primary reformer size and energy demand can be decreased. While at the same time the life and efficiency of the catalysts and the catalyst tubes is increased. This will increase the reaction share of the secondary reformer. By supplying the air in excess to the secondary reformer, the internal firing in the secondary reformer is increased, which makes it possible to achieve the same degree of total reforming as in the case of conventional steam reforming process. The reduced need of energy to the primary reformer will decrease the use of fuel gases and the hence the emissions of combustion flue gases.
Heat Exchange Autothermal Reforming:The heat-exchange autothermal reforming is based upon the principle that the heat of reaction in the secondary reformer can be used to supply the heat needed to the primary reformer for reforming reaction. This eliminates the fired furnace and hence the reformer flue gases, thus significantly reducing the NOx and carbon oxides emissions. Excess air is supplied to the reforming exchanger, to increase the internal firing, which increases the reforming reaction. According to the IFA, these processes can reduce the NOx emissions by 50% or more as compared to the conventional steam reforming processes [2]. Kellogg Brown & Root (formerly M.W. Kellogg Company), introduced its new process in the 1990s, called Kellogg Brown Root Advanced Ammonia Process plus (KAAPplus [11]), which is based upon the same principle.
Improving CO2 Removal Processes:Most of the CO2 removal processes used in the ammonia production use some type of absorbent for CO2 removal. These absorbents have to be regenerated after their use. Those absorbents should be used which are easier to regenerate and do not result in toxic releases. During the regeneration operation proper control of the process can also reduce the waste emissions. Reducing the use of organic absorbents such as amines can also decrease toxic releases to the environment. Use of CO2 removal processes, which do not use any chemicals, is another way of reducing the emissions. Processes such as adsorption and membrane treatment can be used. However, the main problems associated with the membrane processes are the huge pressure drops in the gases after membrane treatment and temperature sensitivity of membranes. The cost of energy to overcome this pressure drop has to be justified before membranes can be used in the ammonia production processes.
Improving Purge Gas Recovery:Improving the purge gas recovery can also control waste emissions. Ammonia is recovered from the synthesis loop purge, while the remaining gases along with small amounts of ammonia are sent to the primary reformer for use as fuel. If the argon present in the purge gas can be removed (i.e. after ammonia removal), then this gas can be recycled back to the converter. Thus increasing the ammonia production.
Better Final Purification Techniques:The main reason for purge requirement in the synthesis loop is the build up of inerts in the recycle stream. Accumulation of these inerts hinders the ammonia conversion in the reactor. The two inert gases present in the feed gas to the ammonia reactor are argon and methane. These inerts keep on accumulating in the recycle stream making it necessary to purge in order to avoid over accumulation of these inerts. If the inert gases can be separated from the ammonia reactor feed gas, then there would be no need for purging. Furthermore, it will also improve the conversion reaction and also decrease the operating cost of the synthesis loop. Rectification columns, Cryogenic purification units, or a combination of both can be used for this purpose. These units can be used prior to the ammonia reactor, after the methanation step.
Ammonia Synthesis:The low conversion rate of the ammonia converters is another reason for waste generation. Most of the conventional ammonia converters (reactors) have a 12-13% conversion. Only recently some reactors have been developed which have conversions as high as 17%. The main reason for this meager conversion is that the ammonia synthesis reaction is highly exothermic. Due to this reason, the temperature of the reaction gases increases as ammonia is formed, increasing the rate of the reaction, but shifting the chemical equilibrium in the opposite direction. Thus the temperature for maximum conversion decreases with the increase in ammonia concentration. With the shift of the reaction in the opposite direction, decomposition of ammonia starts. Also the tremendous amount of heat evolved during the reaction increases the temperature of the catalyst bed to its maximum potential. Further increase in the temperature can permanently damage the catalysts. In order to avoid this situation, the ammonia converters are provided with cooling systems, which remove the heat of reaction. Heat quenching and heat exchanger systems are the two of the many methods used for this purpose. Ammonia synthesis can be improved by improving the converter design. Using improved heat removal techniques and catalyst with higher temperature resistance can improve the ammonia conversion rate. gases increases as ammonia is formed, increasing the rate of the reaction, but shifting the chemical equilibrium in the opposite direction. Thus the temperature for maximum conversion decreases with the increase in ammonia concentration. With the shift of the reaction in the opposite direction, decomposition of ammonia starts. Also the tremendous amount of heat evolved during the reaction increases the temperature of the catalyst bed to its maximum potential. Further increase in the temperature can permanently damage the catalysts. In order to avoid this situation, the ammonia converters are provided with cooling systems, which remove the heat of reaction. Heat quenching and heat exchanger systems are the two of the many methods used for this purpose. Ammonia synthesis can be improved by improving the converter design. Using improved heat removal techniques and catalyst with higher temperature resistance can improve the ammonia conversion rate.
Ammonia Properties1. IdentificationName Commonly used synonyms
C.A.S. Registry number
EINECS Number
EINECS Name
Molecular formula | : :
:
:
:
: | Ammonia Liquid Ammonia
7664-41-7
231-635-3
Anhydrous Ammonia
NH3 |
2. Hazards to Man and the Environment
To manAmmonia is toxic by inhalation and pulmonary oedema may occur up to 48 hours after severe exposure and could be fatal.
Vapours cause irritation and watering of eyes and in the presence of moisture, irritation of the skin.
Liquid ammonia splashes may produce severe burns to the skin and permanent damage to the eyes.
In enclosed spaces (eg vessels, closed workshops) ammonia vapour may be flammable/explosive.
To the environmentAmmonia is toxic to aquatic life.
3. Physical and Chemical Properties| Appearance | Colourless liquefied gas. |
| Odour | Pungent, suffocating. |
| pH water solution (1%) | 11.7 |
| Melting point (triple point) | -77.7°C |
| Boiling point | -33.4°C at 101.3 kPa |
| Explosive limits | 16-27% NH3 by vol. in air at 0 °C |
| Auto-ignition temperature | 651°C |
| Vapour pressure | 1013kPa at 25°C |
| Relative vapour density | 0.6 (air =1) |
| Solubility in water | 529g/l-1 at 20 °C |
| Solubility in organic solvents | Soluble in alcohol, acetone, chloroform |
| Liquid density | 0.6386g/cm-3(at 0°C, 101.3kPa) |
| Gas density | 0.7714g/l-1 (at 0°C, 101.3kPa) |
Envirnomental Data can be found Environmental data for the BAT production
4. ENVIRONMENTAL DATA
Environmental data for the BAT production processes are generally known with a high degree of reliability, while data for storage and transfer are more uncertain and of much less importance. Hence, data for production will be given the highest attention.
4.1 Production Input Requirements4.1.1 >4.1.1 Feedstock The typical feedstock requirements for modern plants are (approximately):-
|
| Conventional reforming: | 22.1 GJ(LHV*).t -1 NH |
| Excess air reforming : | 23.4 GJ(LHV).t -1 NH |
| Autothermal reforming : | 24.8 GJ(LHV).t -1 NH |
| Partial oxidation : | 28.8 GJ(LHV).t -1 NH |
* Lower Heating Value4.1.2 Fuel
Assuming an efficient stand-alone plant with no energy export and no other import than feed-stock and fuel, the fuel requirements are (approximately):-
|
| Conventional reforming: | 7.2-9.0 GJ(LHV).t-1 NH |
| Excess air reforming : | 5.4-7.2 GJ(LHV).t -1NH |
| Autothermal reforming : | 3.6-7.2 GJ(LHV).t-1 NH |
| Partial oxidation : | 5.4-9.0 GJ(LHV).t-1 NH |
The uncertainty in autothermal reforming is mainly due to the type of compressor drives.
4.1.3 Water and air In the steam reforming processes process steam is taken from the plant steam system, usually from an extraction turbine. The net consumption according to the stoichiometic conversion is 0.6-0.7kg.kg
-1 NH
3 , the total supply at a S/C ratio of 3.0 will be about 1.5kg.kg
-1 NH
3 .. In partial oxidation much less steam is fed to the gasification reactor, but additional steam is needed in shift conversion (1.2kg.kg
-1 NH
3 in total).
Process air supply: In conventional reforming the nitrogen supply equals the ammonia nitrogen content plus the purge losses, ie. about 0.85kg N 2 .kg
-1 NH
3 or about 1.1kg air.kg
-1 NH
3 . In the excess air reforming and gas heated reformer cases the process air requirements are about 50% and 100% higher, respectively. In the partial oxidation process the amount of air fed to the air separation unit is approximately 4kg.kg
-1 NH
3 , based on the oxygen requirement.
Boiler feedwater: Assuming all steam condensates are recycled, only the process steam consumption has to be replaced by outside water. This will be 0.7-1.5kg.kg
-1 NH
3 (see above), depending on process condensate recycle or not. Small additional losses and potential import/export have to be allowed for in practice.
Air and/or water for cooling: Will differ from one site to another.
4.1.4 Solvents and additives The consumption of solvent in the CO
2 removal unit should not normally exceed 0.02-0.04kg.t
-1 NH
3 , or about 2kg.h
-1 for a BAT capacity plant. Solvent losses are mainly caused by leaks.
The usual treatment additives and regeneration agents are used in the boiler feedwater preparation units. The consumption figures should not differ from those of a standard steam boiler plant at the same location.
4.1.5 Catalysts
Approximate consumption figures, based on average filling volumes and normally recommended operating periods, for a gas based conventional reforming plant, are given in the table below. The consumptions refer to a capacity of 1,500t.d-1.
|
| Catalyst type | Typical replacement m3.y-1 |
| Hydrodesulphurisation | 1 |
| Sulphur removal | 5 |
| Primary | 5 |
| Secondary reforming | 4 |
| High temperature shift | 10 |
| Low temperature shift | 20 |
| Methanation | 2 |
| Synthesis | 10 |
Actual consumptions in existing plants may differ considerably from the guidance figures above.
4.1.6 Energy requirements The total energy requirement in the reforming BAT processes is 28.8-31.5GJ (LHV).t
-1 NH
3 for a stand-alone plant with no energy export and no other import than feedstock and fuel. When using process waste heat in a gas heated reformer, the process itself will not produce enough steam to drive all the compressors. A part of the power needed may then be imported from a more efficient power plant outside the process plant. In such cases the total energy consumption may be lowered and approach the present practical minimum of 27 GJ(LHV).t
-1 NH
3.
3.
In partial oxidation plants the total energy requirement is 36.9(35.1-37.8)GJ(LHV).t
-1 NH
3 .. This includes imported power and/or auxiliary steam for driving the machinery.
4.2 >4.2 Production Output4.2.1 Ammonia Ammonia production in the typical size BAT plant is 1,000
-1,500t.d
-1 ,500t.d
-1 (300,000-500,000t.y
-1). The production not used in downstream plants on site is stored as described in Chapter 3.
Commercial anhydrous ammonia has two grades of purity:-
- Anhydrous ammonia min. 99.7 wt %, water content (about 0.2% wt)
- Anhydrous ammonia min. 99.9 wt %
4.2.2 Carbon dioxide Carbon dioxide is produced according to the stoichiometric conversion and may be recovered for down-stream uses. The carbon dioxide production in steam/air reforming of natural gas is 1.15-1.30kg.kg
-1 NH
3, dependent on the degree of air reforming. A CO
2 /NH
3 mole ratio of 0.5 (weight ratio 1.29), the stoichiometric ratio for urea production, is obtainable in the heat exchange reformer concepts. In partial oxidation of residual oils the CO
2 production is 2-2.6kg.kg
-1 NH
3 , dependent on feedstock C/H ratio.
Carbon dioxide in the combustion gases is not included in the above figures, but is shown in Figure 3.
4.2.3 Sulphur In BAT partial oxidation most (87-95%) of the sulphur content of the feed to the gasifier is recovered in the Claus unit.
4.2.4 >4.2.4 Steam export Modern steam reforming processes can be designed with no steam export or with some export of low/medium pressure steam if this can be favourably used on site. Steam export is usual in excess air reforming processes where the process air compressor is driven by a gas turbine, and in cases when electric power is used for driving one or more of the main compressors.
Processes with gas heated primary reforming may be designed for zero steam export even with some power import or gas turbine drive.
The partial oxidation process has a steam deficit if all compressors are steam driven.
4.3 Production Emissions and Wastes4.3.1 Emissions into air from steam reforming plants From steam reforming plants with a fired primary reformer emissions into air come from the following sources:-
- Flue-gas from the primary reformer
- Vent gas from CO
2 removal
- Breathing gas from oil buffers (seals/compressors)
- Fugitive emissions (from flanges, stuffing boxes etc.)
- Purge and flash gases from the synthesis section (usually added to the
- primary reformer fuel)
- Non-continuous emissions (venting and flaring)
4.3.1.1 Flue-gas from the primary reformer The flue-gas volume, at 3% (dry gas base) oxygen, for a gas-based conventional steam reforming plant producing 1,500t.d
-1 , is approximately 200,000Nm
3 .h
-1, containing about 8% CO
2 (dry gas base), corresponding to 500kg CO
2.t
-1 NH
3 .. The flue-gas volume from excess air reforming may be lower. The other pollutants in the flue-gas are:-
NO
x : 200-400mg.Nm
-3 , (98-195ppmv), or 0.6-1.3kg.t
-1 NH
3 expressed as NO
2 SO 2 : 0.1-2mg.Nm
-3 ,, (<0.01kg.t
-1) ,, depending on fuel
CO: <10mg.Nm
-3 ,, (<0.03kg.t
-1)
The NO
x emission depends on several factors and the following features reduce the emission:-
- Low combustion air and fuel gas preheat
- Steam/inert injection
- Low ammonia content in injected purge-gas
- Low excess oxygen
- Low NO
x burners
- Post-combustion measures
The SO
2 emission comes from the sulphur in the fuel gas and can be calculated by a simple mass balance.
4.3.1.2 Vent gases from CO2> removal More or less of the CO
2 product may have to be vented, depending on the CO
2 requirements of other production facilities on the site. In some cases, high purity CO
2 is used, while an air-CO
2 mixture from a stripping column is vented. The CO
2 contains small traces of synthesis gas and absorption solvent vapour.
4.3.1.3 Breathing gas from oil buffers This contains traces of NH
3 , synthesis gas and lube oil.
4.3.1.4 >4.3.1.4 Fugitive emissions The diffuse emissions from flanges, stuffing boxes etc. should be minimised.
4.3.1.5 >4.3.1.5 Purge and flash gases The purge and flash gases from the synthesis section are usually washed with water to remove/recover ammonia, and the purge gas may be treated in a recovery unit, before routing the off-gases to the primary reformer fuel gas system. The off-gases are thus combusted and end up as part of the flue-gas. It is important to remove the ammonia as far as possible, as it will contribute considerably to the flue-gas NOx emission.
4.3.1.6 Non-continuous emissions Emission of NOx during flaring synthesis gas at start-up or trip situations is estimated to be 10-20kg.h
-1 as NO
2 [1]. Some plants without a flare, vent to the atmosphere.
4.3.2 >4.3.2 Emissions into air from heat exchange reforming The flue-gas volume (from auxiliary fired equipment) and thus NO
xand CO
2 emissions are considerably reduced, as these processes have much more internal combustion than the fired reformer processes because all the reforming process heat is generated by internal combustion.- Total C0
2 emissions are fixed by the energy consumption. For self-sufficient plants the flue-gas volume in heat exchange reforming is about 50% of the figure for conventional steam reforming. In plants with power import the flue-gas volumes are lower. Reduction of NO
x emissions by 80% has been claimed.
4.3.3 Emissions into air from partial oxidation plants The partial oxidation process has the same emission sources as described for the reforming- process except for the primary reformer flue-gas. A partial oxidation plant may also have auxiliary boiler(s) for power steam production, if more efficiently off-site produced power is not available. The fuel to the auxiliary boiler/superheater together with possible scrubbing equipment determines the amount of CO
2 in the flue-gas. Tail gas from sulphur recovery will also contain sulphur oxides. This means that the CO
2 emission from partial oxidation plants (max 1,500mg.Nm
-3 ) is higher than in the reformer flue-gas. Other additional emissions may be H
2S (0.3ppmv), methanol (max. 100ppmv), CO (30ppmv), and dust (traces, max. 50mg.Nm
-3 ). NO
xemission (max. 700mg.Nm
-3 ) depends on the factors listed for the reformer flue-gas and the nitrogen content of the fuel.
Excess nitrogen is usually vented.
4.3.4 Emissions into water/P>
Pollution problems related to water, during normal operation, may occur due to process condensates or due to the scrubbing of waste gases containing ammonia. In partial oxidation, soot and ash removal may cause pollution problems, if not properly handled.
Process condensate is found primarily in the condensation section prior to the CO
2- removal, of the order of 1m 3 per ton NH
3produced. Without treatment this condensate can contain up to 1kg of ammonia and 1kg methanol per m
3 . More than 95% of the dissolved gases can be recovered by stripping with process steam and are recycled to the process.
The stripped condensate can be re-used as boiler feedwater make-up after treatment by ion exchange. Total recycle is obtained in this way. In some cases the process condensate is used for feed-gas saturation and thus recycled to the process.
Usually the ammonia absorbed from purge and flash gases is recovered in a closed loop so that no aqueous ammonia emissions occur. Emissions into water from the production plant during normal operation can thus be fully avoided.
Soot from gasification in partial oxidation processes is usually recovered and recycled to the process. Traces of soot and slag are emitted to water.
4.3.5 Solid wastes The BAT ammonia processes do not normally produce solid wastes. Spent catalysts and mol.sieves have to be removed and valuable metals are recovered from them. In partial oxidation plants sulphur is recovered in the Claus plant and can be used as feedstock in sulphuric acid units. The ash can be upgraded and used as an ore substitute.
4.4 Environmental Data for Ammonia Storage and Transfer In a refrigerated storage, the cold losses are balanced by recompressing and recondensing the evaporated ammonia. During recompression, some inerts containing also traces of ammonia cannot be condensed but must be flared or scrubbed with water. Small continuous emissions may thus occur, in addition to minor non-continuous emissions during loading operations.
4.5 Environmental Hazards Associated with Emissions and Wastes The production of ammonia is relatively clean compared to many other chemical processes. During the normal operation of a reforming plant, only the NO
x and CO
2- emissions have to be considered. In partial oxidation plants with oil-fired auxiliary boilers the reduction of SO
2 - emissions can be achieved by using low sulphur fuel oil. Generally the emissions from modern ammonia plants have little environmental impact.
4.6 Emission Limits and Guideline Values for Ammonia Production in Some West-European Countries Two types of emission values are of importance:-
- Legally binding emission limit values for specific pollutants which apply for
- ammonia production
- Guideline values which are not legally binding but provide the background for
- requirements laid down in individual permits
Specific legally binding emission limits for ammonia production are only laid down in Germany. In the Netherlands and in Germany limits for emissions from boilers have been laid down which include chemical reactors.
Specific emission guideline values are laid down in the United Kingdom.
With regard to the other countries, no national emission limits or guidelines are fixed for ammonia production plants. Very often the values are the subject of negotiations between the operator and the authority responsible for granting licences. In some countries, these authorities are of local character, so that even within one and the same country, different air pollution requirements may apply for comparable plants. At least in the Benelux countries and Ireland, the licensing authorities take into account emission limits applied in other countries, in particular those laid down in Dutch and German law, in their negotiations with operators. In practice, these values play the role of guideline values.
Indirectly, the emission limits laid down for combustion installations play an important role because the energy consumption of an ammonia plant is relatively high. The emissions from a fired reformer or auxiliary boilers should conform with emission limits and guideline values adopted by the Council of EC in Directive 88/609/EEC or applied by the state.
More detailed information is given in Reference [1].
7. OCCUPATIONAL HEALTH & SAFETY< The occupational health and safety issues associated with ammonia production and storage are:-
- Fire/explosion injuries
- Poisoning
- Suffocation
Fires or explosions from the involuntary ignition of leaks are credible, especially when these occur in the feed-gas and synthesis gas systems (hydrocarbons, hydrogen).
The most important toxic components are CO from potential leaks in the synthesis gas generation and shift areas and NH
3 from leaks in the ammonia synthesis and ammonia handling areas. In partial oxidation plants H
2S and SO
2 are present in the sulphur removal/recovery sections. Traces of carbonyls (iron and nickel) may form during operation.
Suffocation due to lack of oxygen may occur at points where the breathing air has been diluted with inert gases. In ammonia plants CO
2 and N
2 are potentially suffocating gases.
ACGIH [4] occupational exposure limits for ammonia and other components associated with ammonia production are given in the table below. All the figures are ppmv:-
| Component | TLV-TWA (8hr) | TLV-STEL (15min) |
| NH3 | 25 | 35 |
| NO2 | 3 | 5 |
| SO2 | 2 | 5 |
| H2 S | 10 | 15 |
| CO | 50 | 400 |
| CO2 | 5,000 | 30,000 |
The figures are subject to updating and may vary between European countries.
Full health and safety information is given in Safety Data Sheets which must be available and updated. General product information on ammonia is given in Appendix 2.
Ammonia plants have high technological standards and need professional management, operating and maintenance working routines and personnel. Precautions taken to prevent accidents and injuries during operation are incorporated in the operating and safety procedures for the plant.
8. SUMMARY OF BAT EMISSION LEVELS
8.1 Achievable Emission Levels for New Plants<
The following emission levels can be achieved for new ammonia plants. These levels relate to steady-state production, not accounting for peaks which can occur during the unsteady transient conditions of start-up and shut-down and during emergencies.
8.1.1 Emissions into air | ppmv | mg.Nm-3 | kg.t-1 of product |
| NOx (as NO2 at 3% O2) | 75 | 150 | 0.45 |
| SO2 (partial oxidation plants): as for combustion plants. |
8.1.2 Emissions into water<
NH
3 or NH
4 (as N) 0.1kg.t
-1 of product
8.1.3 Waste material<
Spent catalysts etc. <0.2kg.t
-1 of product
Reclaiming of raw materials from spent catalyst by specialised companies prior to their disposal is common practice in the ammonia industry.
In new reforming plants the total energy consumption should not exceed 29.3GJ(LHV).t
-1 and process condensate recycle should be a pre-requisite.
8.2 Achievable Emission Levels for Existing PlantsThe following emission levels can be achieved for existing ammonia plants. As for new plants, these levels do not account for peak values during unsteady or transient conditions. Contributions from ammonia-containing purge gases can be excluded from these figures in special cases, when authorised locally.
8.2.1 Emissions into air<
| ppmv | mg.Nm-3 | kg.t-1 of product |
| NOx (as NO2 at 3% O2) | 150 | 200-400 | 0.9 |
| SO2 (partial oxidation plants): as for combustion plants. |
8.2.2 Emissions into waterNH
3 or NH
4 (as N) 0.1kg.t
-1 of product
8.2.3 Waste materialSpent catalysts etc. <0.2kg.t
-1 of product
8.3 Cost of Pollution Control MeasuresThe costs of pollution control measures in the fertilizer industry are difficult to generalise as they depend on a number of factors such as:-
- The emission target or standard to be met
- The type of process, the degree of integration with other processes on site,
production volumes,
type of raw materials being used etc.
- Whether the plant is new so that the design can be optimised with respect to
pollution abatement, or whether the plant is an existing one requiring revamping or
end-of-pipe pollution abatement equipment
Generally, it is more economic to incorporate the pollution abatement system at the process design stage rather than revamping or "adding-on" equipment at a later stage.
Process condensate recycle can be included to bring existing plants to BAT standards for emissions into water and a rough investment of the order of 2.9 to 3.3 million EUR can be anticipated (1,500t.d
-1).<
5. EMISSION MONITORING<
As outlined in Chapter 4, the emissions of pollutants to be expected from ammonia production are mainly:-
- CO
2, NO
x, SO
2, and CO in the flue-gas from steam reforming processes with a fired
primary reformer and/or auxiliary boilers
- SO
2 from the sulphur removal and recovery systems in partial oxidation processes
- CO
2 and traces of removal solvents and synthesis gas in vent streams from the CO
2 removal system
- Small non-continuous and fugitive emissions
- NH
3 and methanol in process condensates (if not recycled)
The following emissions into air should be monitored as part of a proper supervision:-
- NO
x in flue-gases
- SO
2 in flue-gases (may be calculated by mass balance instead of monitoring
emission, if S input is known)
- SO
2 and H
2S from sulphur removal/recovery systems in partial oxidation processes
The other emissions into air need not be monitored. CO
2 emission can be calculated from fuel specification and energy consumption, CO emission is fixed by the operating conditions and usually stable and low. Non-continuous and fugitive emissions are difficult to measure.
The frequency of monitoring depends on local circumstances and the operating stability of the actual plant. Under normal operating conditions, measurements once a month are usually sufficient.
Methods for discontinuous and continuous measurements of NO
x, SO
2 and H
2S are available and are to a large extent standardised at national level (Norme Belge/Belgische norm, British Standard, AFNOR, Verein Deutscher Ingenieure, Nederlands Normalisatie Instituut). Chemiluminescence or photometry are the most widely used methods for NO
x. SO
2 is determined by Infra Red (IR) absorption techniques. Traces of H
2S are measured by lead acetate.
Emissions into water from new plants are virtually zero as process condensates are recycled and monitoring is not normally required. In existing plants without recycle of process condensate, the ammonia and methanol content should be monitored.
A description of available methods for monitoring emissions is given in Appendix 1.





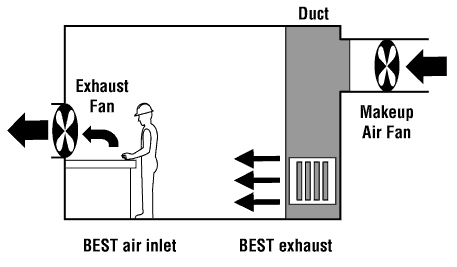
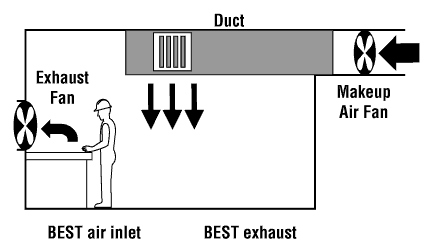
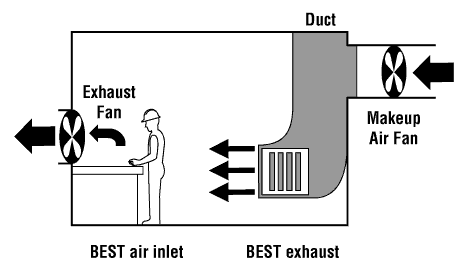
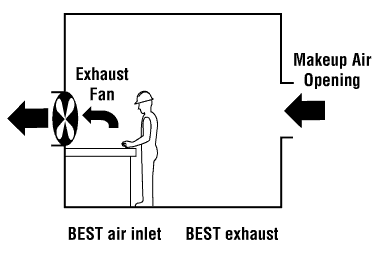
















 World Health Organisation (WHO) thresholds.
World Health Organisation (WHO) thresholds.










Add a comment