Ion exchange capacity
Introduction
Ion exchange is a cyclic process: ions are loaded to resins, the resins get progressively exhausted, and when there is no place to load more ions, the loading phase is interrupted, and the resins must be regenerated.Ion exchange capacity indicates the quantity of ions loaded to the resin.
Definitions
Total capacity
The total capacity of a resin sample is the number of ion exchange sites.In other words, the total capacity is the maximum theoretical quantity of ions that the resin can load.Operating capacity
Also called useful capacity, it is the number of ion exchange sites where exchange has really taken place during the loading run. It is also the number of resin charges — not the number of ions because some ions have more than one charge — picked up by the resin in one cycle. In other words, the operating capacity is the actual quantity of ions loaded on the resin between regenerations.The ion exchange capacity is expressed as eq/L (equivalents per litre of resin).
The unit of mole should be avoided altogether in ion exchange, as it does not take valence into account and brings only confusion. For reference: 1 eq = 1 mole / valence.
The operating capacity is always smaller than the total capacity. We will see why.
Zone of exchange
Ideal case
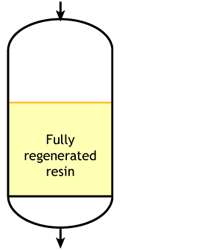 Start of the run | 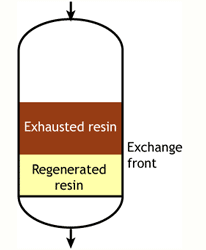 Middle of the run | 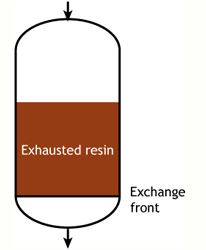 End of the run |
In the real world, there are two cases:
Case 1: the resin is totally regenerated at the beginning of the run (WAC & WBA)
 Start of the run | 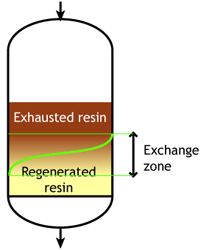 Middle of the run | 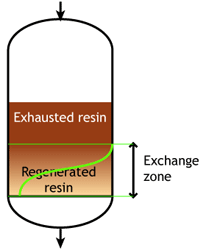 End of the run |
At some stage, the ions begin to "leak" iinto the treated water. The loading run is stopped at the time the concentration of this ion leakage reaches a preset value. This is called the endpoint of the run. At this stage, the ion exchange resin is not fully exhausted, so the operating capacity is smaller than the total capacity.
The operating capacity is, as defined above, the difference between the exhausted resin at the start and at the end of the run. The behaviour shown here is typical of weakly acidic and weakly basic ion exchange resins, that can be fully regenerated with a minimum amount of regenerant, close to the stoichiometric value. A stoichimetric regenerant quantity is the quantity of chemical equivalents exactly equal to the ionic load during the exchange cycle. In practice, weak resins are regenerated with a small excess over the stoichiometric quantity.
The typical operating capacity of a weak base anion exchange resin is 70 to 90 % of the total capacity. For weak acid cation resin, operating capacity depends on several parameters, so there is no such simple estimate. However, WAC resins having a high total capacity and being regenerated almost without an excess (see regenerant ratio), their use is very helpful for waters containing a high concentration of alkalinity and hardness.
Case 2: the resin is partially exhausted at the beginning of the run (SAC & SBA)
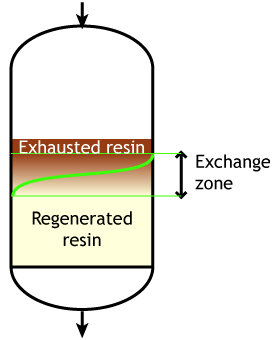 Start of the run | 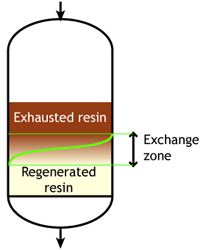 Middle of the run | 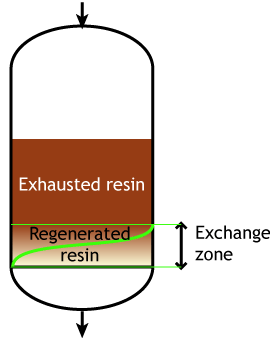 End of the run |
Typically the operating capacity of SAC and SBA resins is 40 to 60 % of their total capacity.
Case 2b: co-flow regenerated resins
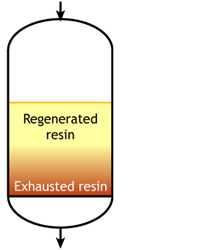 Start of the run | 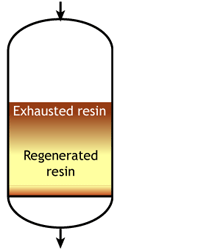 Middle of the run |  End of the run |
For example, if the resin is regenerated with acid, some of the H+ ions released by the removal of Na+ ions from the feed wander down the column and displace a few of the Na+ ions left at the bottom after the previous regeneration. The sodium leakage is thus much higher than with reverse flow regeneration.
Ion exchange kinetics
Weak acid and weak base resins are sensitive to flow rate. When the flow rate increases, the reaction zone becomes longer.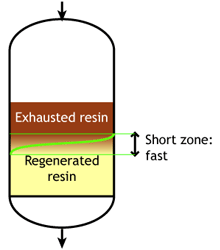 Low flow rate: the reaction zone is short | 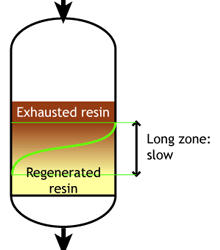 High flow rate: the reaction zone is long |
SAC and SBA resins are less sensitive to flow rate.
Fine resins have generally higher kinetics; this is especially true for WAC and WBA resins. The reason is a shorter path for the ions to travel inside the resin beads.
Parameters affecting operating capacity
The operating capacity depends on a number of process variables including:- Concentration and type of ions to be adsorbed
- Service flow rate
- Temperature
- Type, concentration and quantity of regenerant
- Type of regeneration process (co-flow, reverse flow...)
- Bed depth (reverse flow regeneration only)
- Particle size of the ion exchange resins
Measurement of the total capacity
The total capacity of a resin sample is measured by titration and expressed in eq/L. The procedure involves a volume measurement and must be carried out under strict conditions. As the volume changes according to the ionic form of the resin — some ions have a higher mass and their volume is different from others — the ionic form of measurement must always be reported. The total capacity must also be reported as dry weight capacity after drying of the resin sample. The dry weight capacity measures the number of active groups per kg of dry resin, i.e. without the moisture content. It is expressed in eq/kg. Mention of the ionic form is critical here as well, as different ions have different masses.Dry weight capacity is important for two different purposes:
- For new resins, it gives information about the efficiency of the activation process: for instance, if every aromatic ring has been sulphonated in a strongly acidic resin, the theoretical maximum total dry weight capacity is about 5.5 eq/kg in H+ form.
- For used resins, it gives information about a possible fouling: a fouled resin sample contains foreign matter, which increases the dry weight, and as a consequence the dry weight capacity (number of active groups per kg of dry matter) decreases, even if no functional group has been lost.
Operating capacity in practice
We have seen that the operating capacity of an ion exchange resin is a fraction of the total capacity. It is also expressed in eq/L (equivalents per litre of resin) and indicates the quantity of ions (more precisely the number of charges) that can be exchanged during a cycle. The following table shows typical total and operating capacity values for the common resins (all values in eq/L, most common value in brackets):| Resin type* | Total capacity | Operating capacity |
|---|---|---|
| WAC | 3.7 to 4.5 [4.2] | 1.0 to 3.5 |
| SAC | 1.7 to 2.2 [2.0] | 0.6 to 1.7 |
| WBA | 1.1 to 1.7 [1.3] | 0.8 to 1.3 |
| SBA | 0.9 to 1.4 [1.2] | 0.4 to 0.9 |
Each litre of ion exchange resin will thus be able to treat 1100 / 4.4 = 250 litres of the hard water before having to be regenerated. In ion exchange jargon, this means that the throughput is 250 bed volumes. If the water hardness is higher, the throughput will be less, and vice-versa. See also concentration and capacity units.
Experimental calculation of the operating capacity
Softening example
You have an ion exchange column containing a volume V (litres) of SAC(Na) resin. The water you are treating contains a concentration C of hardness expressed in meq/L. To measure and calculate the operating capacity, you must measure continuously the residual hardness coming out of the column, or take a sample every few minutes (say 5 to 15 min) and analyse its hardness. Plotting the individual residual hardness values should produce the following red curve.
R = Q · C
And the operating capacity of your column is: Cap = Q · C / V (meq/L)
Example: your column holds V = 2000 L of resin (Amberjet 1000 Na), your feed water contains C = 6 meq/L of hardness, your throughput Q is 360 m3 = 360'000 L, so the operating capacity of the column is Cap = (360'000 · 6) / 2000 = 1080 meq/L = 1.08 eq/L
Exactly the same calculation would apply to a nitrate removal column using a nitrate-selective SBA resin, where the nitrate concentration would be used instead of hardness. Demineralisation
The situation here is more complicated, because you have at least two resin types (cation and anion exchangers), and sometimes three or four (weak and strong). If you have two resins only, the cation resin profile is similar to the above graph, but you would use the conductivity (after the anion column) as a control parameter instead of hardness. To calculate the cation resin capacity, you would need the total cation concentration in the feed water, and for the anion resin you would need the anion concentration after degasifier (if any) making sure to add silica and free CO2 to the anions.Important remark
The test should not be done with a "virgin" resin. A few cycles (typically two or three) must be performed before the system reaches an equilibrium. The capacity of the first run (called cycle zero) is higher than that obtained in the subsequent cycles, because in this first run the resin is totally regenerated, which is not the case later.| ION EXCHANGE | Update 14 Jun 2013 |
Abbreviations and units
used in these pages
| Abbreviation | Description |
|---|---|
| Alk | Alkalinity (essentially HCO3) |
| BOD | Biological oxygen demand |
| BV | Bed volume (1 BV = 1 m3 water per m3 resin) |
| BV/h = h–1 | Specific flow rate or space velocity (1 BV/h = 1 m3 water per m3 resin per hour) |
| CFR | Co-flow ("co-current") regeneration |
| COD | Chemical oxygen demand (oxidisable organics) |
| DI | De-ionisation |
| DM | Demineralisation (is the same as DI) |
| DVB | Divinylbenzene (cross-linker) |
| EMA | Equivalent mineral acidity (= Cl + SO4 + NO3 in raw water) |
| eq | Equivalent (= mole divided by valency) eq/L is a unit of ion exchange capacity, meq/L is a unit of ionic concentration |
| FB | Free base (regenerated) form of a WBA resin |
| FMA | Free mineral acidity (= Cl + SO4 + NO3 after cation exchange) |
| IX | Ion exchange |
| kPa | Kilopascal (unit of pressure = 0.01 bar) |
| MB | Mixed bed |
| meq | Milliequivalent (see eq) |
| ppb | Part per billion (10–9 = µg/kg or µg/L or mg/m3) (very dilute solutions) |
| ppm | Part per million (10–6 = mg/kg or mg/L or g/m3) (in dilute solutions) |
| ppt | Part per trillion (10–12 = ng/kg or ng/L or µg/m3) (extremely dilute solutions) |
| RFR | Reverse flow (counterflow or "counter-current") regeneration |
| RO | Reverse osmosis |
| SAC | Strong acid cation [resin] |
| SBA | Strong base anion [resin] |
| TDS | Total dissolved solids |
| TH | Total hardness (alkaline earth metals) |
| TOC | Total organic carbon |
| WAC | Weak acid cation [resin] |
| WBA | Weak base anion [resin] |
| µS/cm | MicroSiemens per centimetre (unit of conductivity) |