Hazard Control
- What is a hazard control program?
- How do I know what kind of control is needed?
- Why should a workplace implement hazard controls?
- What are the main ways to control a hazard?
- Where are controls used?
- What is meant by elimination?
- What is substitution?
- What are examples of engineering controls?
- What are examples of administrative controls?
- What should I know about personal protective equipment (PPE) as a hazard control method?
- Why is it important to monitor and review your hazard control program and methods?
What is a hazard control program?
A control program consists of all steps necessary to protect workers from exposure to a substance or system, and the procedures required to monitor worker exposure and their health to hazards such as chemicals, materials or substance, or other types such as noise and vibration. A written workplace hazard control program should outline which methods are being used to control the exposure and how these controls will be monitored for effectiveness.
How do I know what kind of control is needed?
Selecting an appropriate control is not always easy. It often involves doing a risk assessment to evaluate and prioritize the hazards and risks. In addition, both "normal" and any potential or unusual situations must be studied. Each program should be specially designed to suit the needs of the individual workplace. Hence, no two programs will be exactly alike.
Choosing a control method may involve:
- evaluating and selecting temporary and permanent controls
- implementing temporary measures until permanent (engineering) controls can be put in place
- implementing permanent controls when reasonably practicable
For example, in the case of a noise hazard, temporary measures might require workers to use hearing protection. Long term, permanent controls might use engineering methods to remove or isolate the noise source.
Why should a workplace implement hazard controls?
Some hazards and their controls will be specifically outlined in legislation. In all cases, the employer has a duty of due diligence and is responsible for 'taking all reasonable precautions, under the particular circumstances, to prevent injuries or accidents in the workplace'.
In situations where there is not a clear way to control a hazard, or if legislation does not impose a limit or guideline, you should seek guidance from occupational health professionals such as an occupational hygienist or safety professional about what is the "best practice" or "standard practice" when working in that situation.
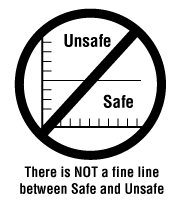
![There is NOT a fine line between safe and unsafe]()
Figure 1
A legal limit or guideline (such as an exposure limit) should never be viewed as a line between "safe" and "unsafe". The best approach is to always keep exposures or the risk of a hazard as low as possible.
What are the main ways to control a hazard?
The main ways to control a hazard include:
- Elimination (including substitution): remove the hazard from the workplace.
- Engineering Controls: includes designs or modifications to plants, equipment, ventilation systems, and processes that reduce the source of exposure.
- Administrative Controls: controls that alter the way the work is done, including timing of work, policies and other rules, and work practices such as standards and operating procedures (including training, housekeeping, and equipment maintenance, and personal hygiene practices).
- Personal Protective Equipment: equipment worn by individuals to reduce exposure such as contact with chemicals or exposure to noise.
These methods are also known as the "hierarchy of control" because they should be considered in the order presented (it is always best to try to eliminate the hazard first, etc).
Where are controls used?
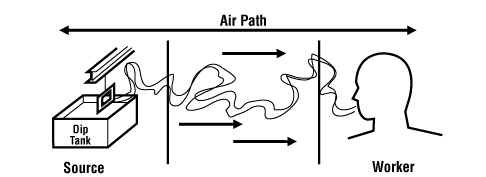
Controls are usually placed:
- At the source (where the hazard "comes from")
- Along the path (where the hazard "travels")
- At the worker
![Controls are place at the source, along the path, and at the worker]()
Figure 2
Control at the source and control along the path are sometimes also known as engineering controls (see below for more details)
What is meant by elimination?
Elimination is the process of removing the hazard from the workplace. It is the most effective way to control a risk because the hazard is no longer present. It is the preferred way to control a hazard and should be used when ever possible.
What is substitution?
Substitution occurs when a new chemical or substance is used instead of another chemical.It is sometimes grouped with elimination because, in effect, you are removing the first substance or hazard from the workplace. The goal, obviously, is to choose a new chemical that is less hazardous than the original.
The table below provides some examples:
| Instead Of: | Consider: |
|---|
| carbon tetrachloride (causes liver damage, cancer) | 1,1,1-trichloroethane, dichloromethane |
| benzene (causes cancer) | toluene, cyclohexane, ketones |
| pesticides (causes various effects on body) | "natural" pesticides such as pyrethrins |
| organic solvents (causes various effects on body) | water-detergent solutions |
| leaded glazes, paints, pigments (causes various effects on body) | versions that do not contain lead |
| sandstone grinding wheels (causes severe respiratory illness due to silica) | synthetic grinding wheels such as aluminium oxide |
Remember, however, that you need to make sure the substitute chemical or substance is not causing any harmful effects, and to control and monitor exposures to make sure to the replacement chemical or substance is below occupational exposure limits.
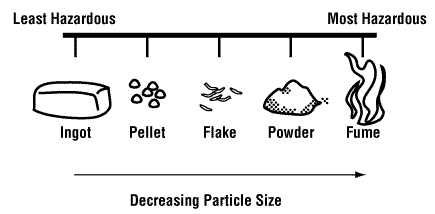
Another type of substitution includes using the same chemical but to use it in a different form. For example, a dry, dusty powder may be a significant inhalation hazard but if this material can be purchased and used as pellets or crystals, there may be less dust in the air and therefore less exposure.
![Decrease particle size]()
Figure 3
When substituting, be very careful that one hazard is not being traded for another. Before deciding to replace a chemical/substance with another, consider all the implications and potential risks of the new material.
What are examples of engineering controls?
Engineering controls are methods that are built into the design of a plant, equipment or process to minimize the hazard. Engineering controls are a very reliable way to control worker exposures as long as the controls are designed, used and maintained properly. The basic types of engineering controls are:
- Process control,
- Enclosure and/or isolation of emission source, and
- Ventilation.
Process Control
Process control involves changing the way a job activity or process is done to reduce the risk. Monitoring should be done before and as well as after the change is implemented to make sure the changes did result in lower exposures.
Examples of process changes include to:
- Use wet methods rather than dry when drilling or grinding. "Wet method" means that water is sprayed over a dusty surface to keep dust levels down or material is mixed with water to prevent dust from being created.
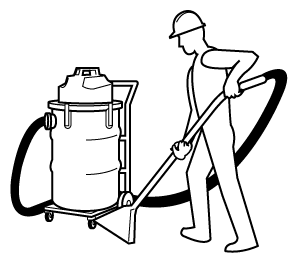
- Use an appropriate vacuum or "wet method" instead of dry sweeping (e.g. with a broom) to control dust and reduce the inhalation hazard.
- Note: Never use a regular "household" vacuum cleaner, especially when cleaning toxic material such as lead, or asbestos. Use a vacuum specifically designed for industrial workplaces and be sure to use appropriate filters, etc.
- Use steam cleaning instead of solvent degreasing (but be sure to evaluate the potential high temperature hazard being introduced such as heat stress).
- Use electric motors rather than diesel ones to eliminate diesel exhaust emissions.
- Float "balls" on open-surface tanks that contain solvents (e.g. degreasing operations) to reduce solvent surface area and to lower solvent loss.
- Instead of conventional spray painting, try to dip, paint with a brush, or use "airless"spray paint methods. These methods will reduce the amount of paint that is released into the air.
- Decrease the temperature of a process so that less vapour is released.
- Use automation - the less workers have to handle or use the materials, the less potential there is for exposure.
- Use mechanical transportation rather than manual methods.
Enclosure & Isolation
These methods aim to keep the chemical "in" and the worker "out" (or vice versa).
An enclosure keeps a selected hazard "physically" away from the worker. Enclosed equipment, for example, is tightly sealed and it is typically only opened for cleaning or maintenance. Other examples include "glove boxes" (where a chemical is in a ventilated and enclosed space and the employee works with the material by using gloves that are built in), abrasive blasting cabinets, or remote control devices. Care must be taken when the enclosure is opened for maintenance as exposure could occur if adequate precautions are not taken. The enclosure itself must be well maintained to prevent leaks.
Isolation places the hazardous process "geographically" away from the majority of the workers. Common isolation techniques are to create a contaminant-free booth either around the equipment or around the employee workstations.
Ventilation
Ventilation is a method of control that strategically "adds" and "removes" air in the work environment. Ventilation can remove or dilute an air contaminant if designed properly. Local exhaust ventilation is very adaptable to almost all chemicals and operations. It removes the contaminant at the source so it cannot disperse into the work space and it generally uses lower exhaust rates than general ventilation (general ventilation usually exchanges air in the entire room).
Local exhaust ventilation is an effective means of controlling workplace exposures but should be used when other methods (such as elimination or substitution) are not possible.
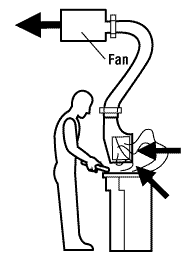
A local exhaust ventilation system consists of these basic parts:
- A hood that captures the contaminated air at the source;
- Ductwork that carries the contaminated air away from the source;
- A fan which draws the air from the hood into the ducts and removes the air from the workspace.
- Air cleaning devices may also be present that can remove contaminants such as dust (particulates), gases and vapours from the air before it is discharged or exhausted into the environment (outside air), depending on the material(s) being used in the hood.
![Air cleaning device]()
Figure 4
The design of a ventilation system is very important and must match the particular process and chemical or contaminant in use. Expert guidance should be sought. It is a very effective control measure but only if it is designed and maintained properly.
Because contaminants are exhausted to the outdoors, you should also check with your local environment ministry or municipality for any environmental air regulations or bylaws that may apply in your area.
What are examples of administrative controls?
Administrative controls limit workers' exposures by scheduling shorter work times in contaminant areas or by implementing other "rules". These control measures have many limitations because the hazard itself is not actually removed or reduced. Administrative controls are not generally favoured because they can be difficult to implement, maintain and are not a reliable way to reduce exposure. When necessary, methods of administrative control include:
- Scheduling maintenance and other high exposure operations for times when few workers are present (such as evenings, weekends).
- Using job-rotation schedules that limit the amount of time an individual worker is exposed to a substance.
- Using a work-rest schedule that limits the length of time a worker is exposure to a hazard.
Work Practices
Work practices are also a form of administrative controls. In most workplaces, even if there are well designed and well maintained engineering controls present, safe work practices are very important. Some elements of safe work practices include:
- Developing and implementing standard operating procedures.
- Training and education of employees about the operating procedures as well as other necessary workplace training (including WHMIS).
- Establishing and maintaining good housekeeping programs.
- Keeping equipment well maintained.
- Preparing and training for emergency response for incidents such as spills, fire or employee injury.
Education and Training
Employee education and training on how to conduct their work safely helps to minimize the risk of exposure and is a critical element of any complete workplace health and safety program. Training must cover not only how to do the job safely but it must also ensure that workers understand the hazards of their job. It must also provide them with information on how to protect themselves and co-workers.
Good Housekeeping
Good housekeeping is essential to prevent the accumulation of hazardous or toxic materials (e.g., build-up of dust or contaminant on ledges, or beams), or hazardous conditions (e.g., poor stockpiling).
For more information about workplace housekeeping,
![Good housekeeping]()
Figure 5
Emergency Preparedness
Being prepare for emergencies means making sure that the necessary equipment and supplies are readily available and that employees know what to do when something unplanned happens such as a release, spill, fire or injury. These procedures should be written and employees should have the opportunity to practice their emergency response skills regularly.
Personal Hygiene Practices and Facilities
Personal hygiene practices are another effective way to reduce the amount of a hazardous material absorbed, ingested or inhaled by a worker. They are particularly effective if the contaminant(s) can accumulate on the skin, clothing or hair.
Examples of personal hygiene practices include:
- Washing hands after handling material and before eating, drinking or smoking;
- Avoiding touching lips, nose and eyes with contaminated hands.
- No smoking, drinking, chewing gum or eating in the work areas - these activities should be permitted only in a "clean" area; and
- Not storing hazardous materials in the same refrigerator as food items.
What should I know about personal protective equipment (PPE) as a hazard control method?
Personal protective equipment (PPE) includes items such as respirators, protective clothing such as gloves, face shields, eye protection, and footwear that serve to provide a barrier between the wearer and the chemical or material.
It is the final item on the list for a very good reason. Personal protective equipment should never be the only method used to reduce exposure except under very specific circumstances because PPE may "fail" (stop protecting the worker) with little or no warning. For example: "breakthrough" can occur with gloves, clothing, and respirator cartridges.
No matter which type of PPE is used, it is essential to have a complete PPE program in place.
Why is it important to monitor and review your hazard control program and methods?
It is important to monitor both the hazard and the control method to make sure that the control is working effectively and that exposure to the hazard is reduced or eliminated.
Some tools include physical inspection, testing, exposure assessment, observations, injury and illness tracking, employee feedback/input, occupational health assessment and other methods.
Be sure to answer the following questions:
- Have the controls solved the problem?
- Is the risk posed by the original hazard contained?
- Have any new hazards been created?
- Are new hazards appropriately controlled?
- Are monitoring processes adequate?
- Have workers been adequately informed about the situation?
- Have orientation and training programs been modified to deal with the new situation?
- Are any other measures required?
- Has the effectiveness of hazard controls been documented in your committee minutes?
- What else can be done?