The Chemistry of Water
The polarity of waterWater has a simple molecular structure. It is composed of one oxygen atom and two hydrogen atoms. Each hydrogen atom is covalently bonded to the oxygen via a shared pair of electrons. Oxygen also has two unshared pairs of electrons. Thus there are 4 pairs of electrons surrounding the oxygen atom, two pairs involved in covalent bonds with hydrogen, and two unshared pairs on the opposite side of the oxygen atom. Oxygen is an "electronegative" or electron "loving" atom compared with hydrogen. Water is a "polar" molecule, meaning that there is an uneven distribution of electron density. Water has a partial negative charge (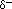 ) near the oxygen atom due the unshared pairs of electrons, and partial positive charges ( ) near the oxygen atom due the unshared pairs of electrons, and partial positive charges ( ) near the hydrogen atoms. ) near the hydrogen atoms. An electrostatic attraction between the partial positive charge near the hydrogen atoms and the partial negative charge near the oxygen results in the formation of a hydrogen bond as shown in the illustration. |  |
The ability of ions and other molecules to dissolve in water is due to polarity. For example, in the illustration below sodium chloride is shown in its crystalline form and dissolved in water. 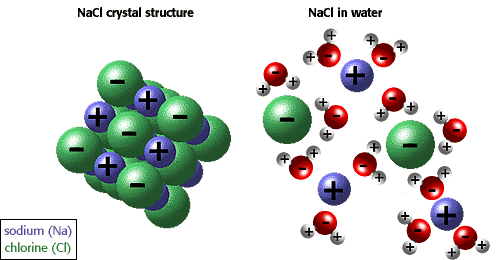 |
Acids and Bases, Ionization of Water
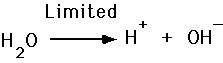
- Acid release H+
- Bases accept H+
- at pH 7.0, a solution is neutral
- at lower pH (1-6), a solution is acidic
- at higher pH (8-14), a solution is basic