What is Nitrogen Dioxide (NO2)?
- NO2: Nitrogen Dioxyde
- NO: Nitric Dioxyde
- NOx: Oxides of Nitrogen = {NO2+NO}
NO2 life-cycle
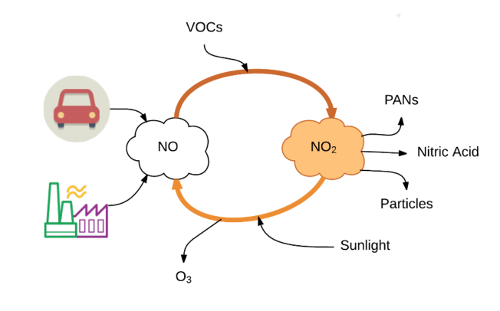
After a few hours in the atmosphere and in the presence of volatile organic compounds (VOCs) the NO is converted to NO2. This reaction can occur over a couple of seconds to a few hours (2).
NO2 reacts further with other substances in the air to form nitric acid, particulate matter and substances called PANs (peroxyacyl nitrates).
Also with sunlight NO2 can convert back to NO and produce ozone (O3) as a side pollutant. Because of the potential of NO2 to produce these "secondary" pollutants it is important to monitor and regulate NO2.
How does NO2 affect me?
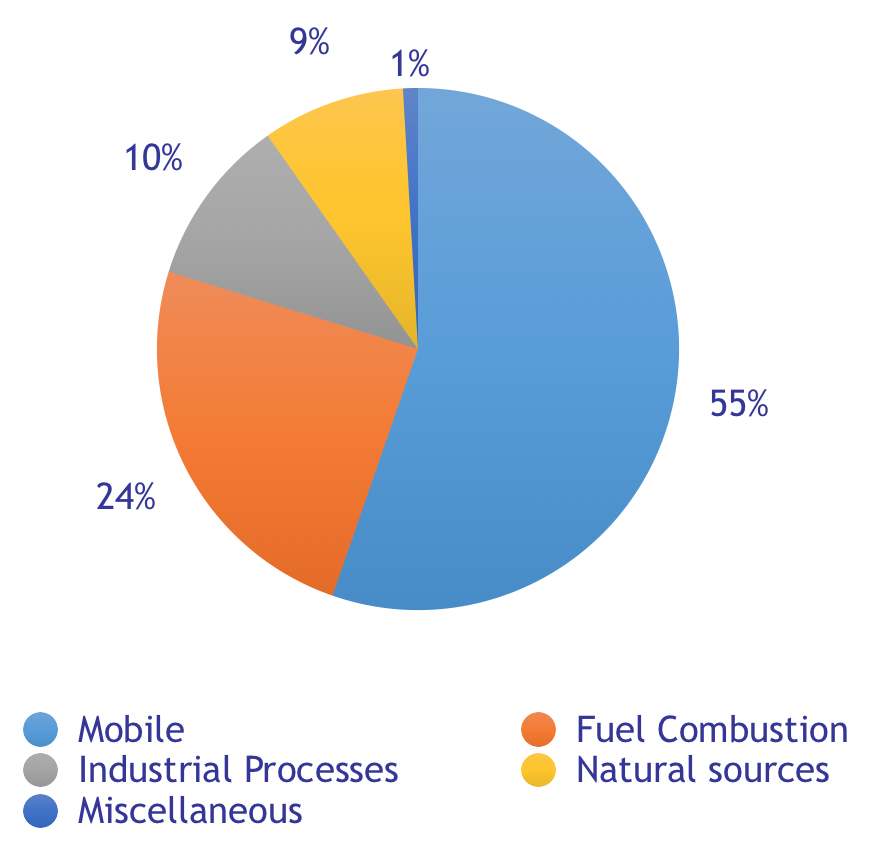
Who and what is emitting NOx?
In areas where road vehicles are the main source of NOx there is often higher NO2concentrations during peak traffic times such as around 5pm weekdays shown in the NO2 AQI below. Some additonal interresting notes:
- The ozone (O3) concentration increases during the day while the NO2concentration decreases. That's because NO2 transforms to NOx in the presence of sunlight.
- After 6 PM, the NO2 concentration builds up as there is no sunlight to convert NO2 back to NO.

Figure 3 Example of AQI in London
Why are NO2 concentration so low?
References and Further Reading
2. Cheremisinoff, Paul N and Young, Richard Alan. Air Pollution Control and Design Handbook. s.l. : M Dekker, 1977. pp. 672-673. Vol. 2.
3. Urban Air Quality in Europe. Boulter, P G, Borken-Kleefeld, J and Ntziachristos, L. [ed.] M Vianna. Berlin Heidelberg : Springer-Verlag, 2013, Handbook of Environmental Chemistry, Vol. 26, pp. 31-54.
4. NOx emissions in China: historical trends and future perspectives. Zhao, B, et al. 13, 2013, Atmospheric Chemistry and Physics, pp. 9869-9897.