Feed water
and some recommended limits for ion exchange systems |
IntroductionIon exchange resins exchange ions. Not a surprise, but the composition of the feed water affects plant performance. It is therefore essential to know precisely the water composition of the feed to the ion exchange system.
The following components and characteristics should be known:
- Salinity (see also the separate page on water analysis details)
- Suspended solids and turbidity
- Temperature
- pH value
- Organic substances in the water
- Other impurities, such as iron, manganese, aluminium, oil, polyelectrolytes...
We will examine the effect of all above parameters and try to set practical limits for each. |
Salinity (water analysis)This is the single most important item to estimate the performance of an ion exchange system. It is also one of the first things to check when plant performance deteriorates. You cannot rely on an analysis that was made months or years ago. Some effects of a change in salinity are:
| Type of change | Effect |
|---|
| Higher salt content | Shorter runs, lower throughput, sometimes lower quality of the treated water | | Lower salt content | Longer runs, higher throughput | | Change in ionic balance (e.g. less bicarbonate, more chloride) | Change in treated water quality. The resin volumes become unbalanced, the degasifier has less or more carbon dioxide to handle | | Higher ratio of silica to total anions | This may increase silica leakage and require a change in regeneration conditions. |
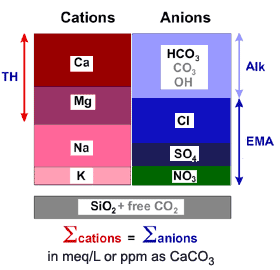
The picture below is a schematic representation of a water analysis, with cations and anions. A good water analysis must be balanced. ![Feed water analysis]() Click on picture to see it enlarged with more details.
If the water analysis varies according to season, plant performance should be re-assessed, and perhaps operating conditions re-adjusted, to reflect the seasonal variations. If you don't analyse the water yourself, give a sample to a reputable laboratory for testing.y.
When re-assessing the performance of a plant, or optimising it, it is recommended to use the most probable analysis for the basic calculation, then to re-run the calculation with seasonal analyses to estimate plant throughput under various conditions. All the water analyses should be real, not maxima, averages or minima.
We strongly recommend that you should update the expected performance of the plant based on actual operating conditions. You should collect the necessary data:
- Water analysis (after pre-treatment)
- Resin types and volumes
- Regeneration method (co-flow, reverse flow, packed beds)
- Regenerant quantities and concentrations
Salinity limitsIon exchange is the perfect technology for low concentrations. At high salinity, the cycles become very short, regenerant consumption increases and in extreme cases the water required for regeneration may exceed the volume of treated water. As a guideline, a salinity of 20 meq/L (1000 ppm as CaCO 3) seems to be the high limit, with some exceptions. Higher salinity water is probably best treated with RO. Sea water cannot be demineralised by ion exchange, as the resins would be exhausted in less than 3 bed volumes. |
Suspended solids and turbidityIdeally, the feed water to an ion exchange vessel should be perfectly clear and free of suspended solids. It is essential to ensure that mechanical filters installed ahead of an ion exchange system operate properly. Insufficient filtration resulting in excessive suspended solids may cause:
- Channeling of the resin bed, resulting in high leakage and short runs.
- High pressure drop values, sometimes resulting in flow reduction, and requiring frequent backwash of the unit.
Suspended solids are traditionally measured by filtration on a 0.45 µm filter and expressed as dry mass. The tolerated amount of suspended solids varies according to the ion exchange technology and to the run length. If the resins can be easily backwashed and cleaned, a higher quantity of suspended solids is acceptable.
- As co-flow regenerated vessels can be backwashed before each regeneration, they are not very sensitive to suspended solids, and several mg/L (ppm) are usually acceptable.
- In all cases, if the system has long cycles, the accumulated suspended solids may cause pressure drop problems even if the amount of suspended solids in the feed is relatively low.
- Reverse flow regenerated vessels are not backwashed at the end of every cycle, and the pressure drop should be monitored closely to determine when a resin backwash is necessary.
- Packed bed units are more sensitive to suspended solids, as they cannot be backwashed in situ. In general, the tolerated suspended solids should be well below 1 mg/L (1 ppm).
- In Upcore plants, the suspended solids land on the surface of the resin bed, and some are backwashed away during regeneration.
- In Amberpack and floating bed, the suspended solids enter in a slightly fluidised part of the bed and accumulate there. A higher quantity is tolerated because it migrates partially upward, but this quantity cannot be removed until the resin is taken out to the backwash tower.
Turbidity (cloudiness or haziness) is measured in NTU (Nephelometric Turbidity Units). There is no fixed relation between turbidity and suspended solids.
Limits for suspended solids There is no simple number here: the most sensible way is to calculate the load of solids during one cycle and to express the result per square metre of vessel (cross-section). Here some suggestions: Suspended solids| System | Max. load per cycle |
|---|
| Co-flow | 6 kg/m2 | | Split-flow | 6 kg/m2 | | RFR hold-down | 2 kg/m2 | | Condensate | 2 kg/m2 | | UpcoreTM& similar | 0.5 kg/m2 | | AmberpackTM& similar | 0.2 kg/m2 | | ADITM, ADNTM | 0.1 kg/m2 |
Turbidity limits Turbidity is not used much in conjunction with ion exchange systems. See suspended solids above. For floating bed systems without a backwash tower, it was found that 1 NTU is more than what the columns can tolerate. |
TemperatureThe temperature of the feed water (and of the regenerants) can affect plant performance.
Some effects of a change in temperature are:
- At low temperature, the operating capacity of all resins decreases.
- There is an exception to the above rule: at high temperature, the silica removal capacity of a SBA resin decreases, to become virtually zero if the temperature exceeds about 60°C.
- Styrenic SBA resins of type 2 (e.g. Amberjet 4600) and acrylic SBA resins (e.g. Amberlite IRA458) should not be operated or regenerated at a temperature higher than 35°C. High temperatures may result in problems of rinse and a loss of strong base capacity, which will cause a higher silica leakage and shorter runs.
- Cation resins can operate at high temperature, sometimes in excess of 100°C. However, the presence of oxygen and trace metals can cause slow oxidation of the resin.
Temperature limits See the table with limits of temperature for all anion exchange resins.
Cation resins can withstand 100°C or even more. Product data sheets give details for all resins. |
pH valueIon exchange resins can tolerate any pH value (0 to 14) without suffering damage, provided strong osmotic shocks due to rapid change of pH or concentration are avoided.
In service however, resins operate only within pH limits: cation resins cannot operate at very low pH, or anion resins at very high pH, because they would be permanently regenerated and unable to exchange other ions. Similarly, the resins are normally not used in very concentrated solutions. This is why in practice the table below should only go up to pH 12 and down to pH 2, which would be 10 meq/L of NaOH or acid respectively.
pH limitsOperating pH range| Type of resin | pH range | | WAC | 6 to 14 | | SAC | 4 to 14 | | WBA | 0 to 7 | | SBA | 0 to 9 | |
OrganicsOrganic matter in water can interfere with ion exchange. The main effect of organics is irreversible fouling of anion exchange resins.
Some problems caused by organics are:
- Low pH (< 6) of the treated water when organic acids slip through the plant.
- High conductivity of the treated water.
- Increased silica leakage.
- Increased time for rinsing and high volume of waste water.
- Shorter runs.
The traditional measurement of organics (COD) in natural water uses the potassium permanganate oxidation method, and its result is expressed in mg/L as KMnO4.
Unfortunately, there is no direct correlation between this method and the more modern analysis of TOC (Total Organic Carbon). However, experience has shown that as a rule of thumb, 1 mg/L TOC (1 ppm as C) can be roughly translated into 5.5 mg/L (5.5 ppm) as KMnO4.
Limits of organic loadSee the table for all anion exchange resins (same as temperature table). |
Other impuritiesOther impurities can also interfere with ion exchange. Some of them are listed below with their effect and possible remedies.
|
| Effects | Prevention/Treatment | Limits |
|---|
| Iron and manganese |
|---|
- Pressure drop
- Short cycles (capacity loss)
- Bad quality (high leakage)
| - Oxidation and filtration
- Resin cleaning with HCl
| Limits for Fe
Softening and nitrate removal: 1 mg/L
Demineralisation HCl: 15 mg/L
Demineralisation H2SO4: 0.5 mg/L
Condensate polishing: 0.1 mg/L (up to 2 mg/L at startup) | | Aluminium |
|---|
- Precipitation of Al(OH)3
(at neutral pH)
| - Al dissolves in acid or alkali
| Limits for aluminium
Aluminium usually does not foul resins unless it is a large proportion of the cationic load. | | Barium |
|---|
| - Regenerate cation resins with HCl only!
| Limits for barium
When Ba is more than 0.1 % of total cations, H2SO4 should be avoided. | | Oil |
|---|
- Short cycles (capacity loss)
- Bad quality (high leakage)
| - Check pumps for oil leakage
- Resin cleaning with non-ionic surfactant
| Limits for oil
Virtually zero
0.05 mg/L maximum | | Oxidants, chlorine or ozone |
|---|
- Short cycles (capacity loss)
- Sodium leakage from anion resins
- Pressure drop when resin gets "soft"
| - Adjust (reduce) dosage
- Use activated carbon as pre-treatment
- Scavenge excess oxidant with bisulphite
| Limits for oxidants
See table with acceptable limits. | | Polyelectrolytes |
|---|
- Short cycles (capacity loss)
- Bad quality (high leakage)
| - Adjust dosage
- Clean resin with 4 % NaOH
| Limits for polyelectrolytes
No known limits. Caution recommended. In doubt, polyelectrolyte supplier should be asked for harmlessness. |
|