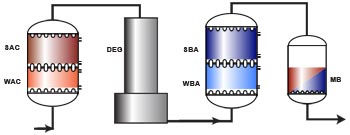
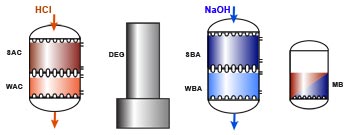
Basic ion exchange processes
in water treatment
Introduction
The ion exchange technology is used for different water treatment applications:- Softening (removal of hardness)
- De-alkalisation (removal of bicarbonate)
- Decationisation (removal of all cations)
- Demineralisation (removal of all ions)
- Mixed bed polishing
- Nitrate removal
- Selective removal of various contaminants
Softening
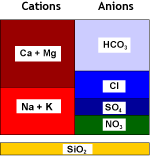
Natural water contains calcium and magnesium ions (see water analysis) which form salts that are not very soluble. These cations, together with the less common and even less soluble strontium and barium cations, are called together hardness ions. When the water evaporates even a little, these cations precipitate. This is what you see when you let water evaporate in a boiling kettle on the kitchen stove.Hard water also forms scale in water pipes and in boilers, both domestic and industrial. It may create cloudiness in beer and soft drinks. Calcium salts deposit on the glasses in your dishwasher if the city water is hard and you have forgotten to add salt.
Strongly acidic cation exchange resins (SAC, see resin types) used in the sodium form remove these hardness cations from water. Softening units, when loaded with these cations, are then regenerated with sodium chloride (NaCl, table salt).
Reactions
Here the example of calcium: 2 R-Na + Ca++![--->]() R2-Ca + 2 Na+
R2-Ca + 2 Na+
R represents the resin, which is initially in the sodium form. The reaction for magnesium is identical.  R2-Ca + 2 Na+
R2-Ca + 2 Na+The above reaction is an equilibrium. It can be reversed by increasing the sodium concentration on the right side. This is done with NaCl, and the regeneration reaction is:
R2-Ca + 2 Na+![--->]() 2 R-Na + Ca++
2 R-Na + Ca++
 2 R-Na + Ca++
2 R-Na + Ca++What happens to the water
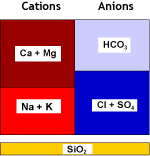 Raw water | SAC (Na) | 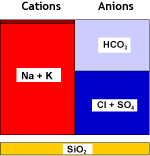 Softened water |
Treated water quality (residual hardness)
| Co-flow regeneration: | Depends on water composition and regenerant level |
| Reverse flow regeneration: | < 0.02 meq/l (1 mg/l as CaCO3) |
Uses
Examples for the use of softeners: - Treatment of water for low pressure boilers
- In Europe, most dishwashers have a softening cartridge at the bottom of the machine
- Breweries and soft drink factories treat the water for their products with food grade resins
De-alkalisation
This particular process uses a weakly acidic cation resin. This resin type is capable of removing hardness from water when it also contains alkalinity. After treatment, the water contains carbon dioxide, that can be eliminated with a degasifier tower. The cation resin is very efficiently regenerated with an acid, usually hydrochloric acid.Reactions
Here the example of calcium: 2 R-H + Ca++(HCO3–)2![--->]() R2-Ca + 2 H+ + 2 HCO3–
R2-Ca + 2 H+ + 2 HCO3–
and the hydrogen cations combine with the birarbonate anions to produce carbon dioxide and water:  R2-Ca + 2 H+ + 2 HCO3–
R2-Ca + 2 H+ + 2 HCO3–H+ + HCO3–![--->]() CO2 + H2O
CO2 + H2O
 CO2 + H2O
CO2 + H2O What happens to the water
 Raw water | WAC (H) | 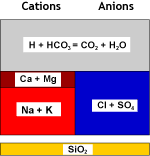 Decarbonated water |
| Recombination of hydrogen and bicarbonate and removal of carbon dioxide with the degasifier: | ||
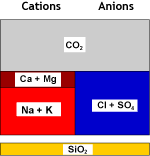 Decarbonated water | DEG | 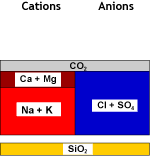 Degassed water |
Treated water quality (after degasifier)
| Temporary hardness | Practically 0 |
| Total hardness | = FMA |
| pH value | 6 to 7 |
Uses
De-alkalisation is used: - In breweries
- In household drinking water filters
- For low pressure boilers
- As a first step before the SAC exchange in demineralisation
Decationisation
The removal of all cations is seldom practiced, except as a first stage of the demineralisation process, or sometimes in condensate polishing where the decationiser precedes a mixed bed unit. A strongly acidic cation exchange resin (SAC) is used in the H+ form.Reactions
Here the example of sodium, but all cations react in the same way: R-H + Na+![--->]() R-Na + H+
R-Na + H+
The equilibrium reaction is reversed for regeneration by increasing the hydrogen concentration on the right side. This is done with a strong acid, HCl or H2SO4:  R-Na + H+
R-Na + H+R-Na + H+![--->]() R-H + Na+
R-H + Na+
 R-H + Na+
R-H + Na+What happens to the water
 Raw water | SAC (H) | 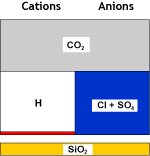 Decationised water | DEG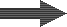 |  Decat + degassed water |
Treated water quality (after degasifier)
If hardness is not zero, there is a malfunction in≤ the system. | Total hardness | 0 |
| Na (co-flow regen.) | 0.5 to 1 mg/L |
| Na (reverse flow regen.) | < 0.1 mg/L |
| pH value | 2 to 5 |
| FMA | Unchanged |
| CO2 | 0.2 to 0.3 meq/L |
Demineralisation
For many applications, all ions in the water must be removed. In particular, when water is heated to produce steam, any impurity can precipitate and cause damage. As there are cations and anions in the water, we must use two different types of resins: a cation exchanger and an anion exchanger. This combined arrangement produces pure water, as presented in the general introduction. Demineralisation is also called deionisation. The cation resin is used in the hydrogen form (H+) and the anion resin in the hydroxyl form (OH–), so that the cation resin must be regenerated with an acid and the anion resin with an alkali.A degasifier is used to remove the carbon dioxide created after cation exchange when the water contains a significant concentration of bicarbonate.
The cation resin is usually located before the anion resin: otherwise if the water contains any hardness, it would precipitate in the alkaline environment created by the OH— form anion resin as Ca(OH)2 or CaCO3, which have low solubility.
Layout SAC – (DEG) – SBA
Let us first consider a simple demineralisation system comprising a strong acid cation exchange resin in the H+ form, a degasifier (optional) and a strong base anion exchange resin in the OH– form. The first step is decationisation as shown above:RSAC-H + Na+![<--->]() RSAC-Na + H+
RSAC-Na + H+
With calcium instead of sodium (also valid for magnesium and other divalent cations):  RSAC-Na + H+
RSAC-Na + H+2 RSAC-H + Ca++![<--->]() (RSAC)2-Ca + 2 H+
(RSAC)2-Ca + 2 H+
In the second step, all anions are removed with the strong base resin:  (RSAC)2-Ca + 2 H+
(RSAC)2-Ca + 2 H+RSBA-OH + Cl–![<--->]() RSBA-Cl + OH–
RSBA-Cl + OH–
The weak acids created after cation exchange, which are carbonic acid and silicic acid (H2CO3 and H2SiO3) are removed in the same way:  RSBA-Cl + OH–
RSBA-Cl + OH–RSBA-OH + HCO3–![<--->]() RSBA-HCO3– + OH–
RSBA-HCO3– + OH–
And finally, the H+ ions created in the first step react with the OH– ions of the second step to produce new molecules of water. This reaction is irreversible:  RSBA-HCO3– + OH–
RSBA-HCO3– + OH–H+ + OH–![--->]() H2O
H2O
 H2O
H2O What happens to the water
| 1: Cation exchange removing all cations (as in decationisation) followed by degassing: | ||||
 Raw water | SAC (H) |  Decationised water | DEG |  Decat + degassed water |
| 2: Anion exchange removing all anions (strong and weak acids): | ||||
 Decat + degassed water | SBA (OH) |  Demineralised water | ||
Treated water quality
Demineralised water is completely free of ions, except a few residual traces of sodium and silica, because the SAC and SBA resins have their lowest selectivity for these. With a simple demineralisation line regenerated in reverse flow, the treated water has a conductivity of only about 1 µS/cm, and a silica residual between 5 and 50 µg/L depending on the silica concentration in the feed and on regeneration conditions. | Conductivity (co-flow regen.) | 5 to 25 µS/cm |
| Conductivity (reverse flow regen.) | < 1 µS/cm |
| Silica residual (co-flow) | 50 to 200 µg/L |
| Silica residual (reverse flow) | 5 to 40 µg/L |
| Sodium residual | See decationisation above |
| pH value | In principle > 7 Don't use pH as a control parameter |
Note that the pH value should not be used as a process control, as it is impossible to measure the pH of a water with less than say 5 µS/cm conductivity.
Regeneration
The SAC resin is regenerated with a strong acid, HCl or H2SO4:
R-Na + H+![<--->]() R-H + Na+
R-H + Na+
And the SBA resin is regenerated with a strong alkali, NaOH in 99 % of the cases:  R-H + Na+
R-H + Na+RSBA-Cl + OH–![<--->]() RSBA-OH + Cl–
RSBA-OH + Cl–
 RSBA-OH + Cl–
RSBA-OH + Cl–Layout WAC/SAC – DEG – WBA/SBA
Because weakly acidic and weakly basic resins offer a high operating capacity and are very easy to regenerate, they are used in combination with strongly acidic and strongly basic resins in large plants. The first step with the WAC resin is dealkalisation (removal of bicarbonate hardness), and the second step with the SAC removes all the remaining cations. A WAC resin is used when both hardness and alkalinity are present in large relative concentrations in the feed water. WBA resins remove only the strong acids after cation exchange. They are not capable of removing the weak acids such as SiO2 and CO2. In the regenerated, free base form, they are not dissociated, so no free OH– ions are available for neutral anion exchange. On the other hand, their basicity is enough to adsorb the strong acids created after cation exchange:RWBA + H+Cl–![--->]() RWBA.HCl
RWBA.HCl
In the last step, a SBA resin is thus required to remove the weak acids, as shown in the preceding section:  RWBA.HCl
RWBA.HCl RSBA-OH + HCO3–![<--->]() RSBA-HCO3– + OH–
RSBA-HCO3– + OH–
 RSBA-HCO3– + OH–
RSBA-HCO3– + OH–What happens to the water
| 1 & 2: Cation exchange beginning with the removal of temporary hardness (WAC, as in dealkalisation) followed by the removal of all remaining cations (SAC): | ||||
 Raw water | WAC (H) |  Decarbonated water | SAC (H) |  Decationised water |
| 3 & 4: Anion exchange begining after degassing with the removal of strong acids (WBA) followed by the removal of weak acids (SBA): | ||||
 Decat + degassed water | WBA (FB) | 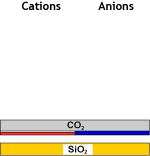 Partially demineralised | SBA (OH) |  Demineralised water |
Regeneration
Regeneration is done in thoroughfare, which means that the regenerant first goes through the strong resin, which requires an excess of regenerant, and the regenerant not consumed by the strong resin is usually sufficient to regenerate the weak resin without additional dosage.
The cation resins are regenerated with a strong acid, preferably HCl, because H2SO4 can precipitate calcium.
The anion resins are regenerated with caustic soda.
The quality obtained is the same as in the simple SAC-SBA layout, but because the weak resins are practicallly regenerated "free of charge", the regenerant consumption is considerably lower. Additionally, the weak resins have a higher operating capacity than the strong resins, so the total volume of ion exchange resins is reduced.
Uses
Examples of demineralisation: - Water for high pressure boilers in nuclear and fossil fuelled power stations and other industries
- Rinse water used in production of computer chips and other electronic devices
- Process water for many applications in the chemical, textile and paper industries
- Water for batteries
- Water for laboratories
Mixed bed polishing
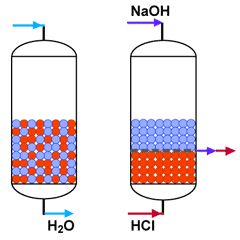
Mixed bed unit in service
and in regeneration
What happens to the water
Practically nothing is left: Demineralised water | SAC (H) + SBA (OH) |  Nothing is left |
The pH value should not be used as a process control, as pH meters are unable to operate at 1 µS/cm conductivity or below.
Treated water quality
| Conductivity | < 0.1 µS/cm |
| Silica residual | 1 to 10 µg/L |
| pH value | Cannot be measured |
Uses
- Treatment of water pre-demineralised with ion exchange resins
- Polishing of reverse osmosis permeate
- Polishing of sea water distillate
- Treatment of turbine condensate in power stations
- Treatment of process condensate in various industries
- Production of ultra-pure water for the semiconductors industry
- Service de-ionisation (with off-site regenerated columns)
Nitrate removal
Nitrate can be removed selectively from drinking water using strong base anion resins in the chloride cycle, i.e. regenerated with a NaCl brine. The reaction is:RSBA-Cl + NO3–![<--->]() RSBA-NO3 + Cl–
RSBA-NO3 + Cl–
 RSBA-NO3 + Cl–
RSBA-NO3 + Cl–What happens to the water
 Raw water | SBA (Cl) |  Denitrated water |
Treated water quality Depends on water composition, regenerant level and mode of regeneration.
Use reverse flow regeneration only. Expected NO3 leakage with RFR: 2 to 10 mg/L.
Use IXCalc for accurate estimates. A high sulphate background will increase leakage.
Use reverse flow regeneration only. Expected NO3 leakage with RFR: 2 to 10 mg/L.
Use IXCalc for accurate estimates. A high sulphate background will increase leakage.
Uses
- Mainly municipal water treatment
Selective removal of various other contaminants
Selective removal of metals and other contaminants is mainly used for drinking water and for waste. Many of these applications require special resins: chelating resin making stable metal complexes, for instance.Examples
- Removal of boron (boric acid) from drinking water
- Removal of nitrate from drinking water (shown above)
- Removal of perchlorate from drinking water
- Removal of heavy metals from waste: Cd, Cr, Fe, Hg, Ni, Pb, Zn
Other information
AbbreviationsResin types are usually abbreviated in these pages:
- SAC: strongly acidic cation exchange resin
- WAC: weakly acidic cation exchange resin
- SBA: strongly basic anion exchange resin
- WBA: weakly basic anion exchange resin