Mineral nutrition
The process involving the absorption and utilization of various minerals ions by plants for their growth and development is called mineral nutrition.
Chemical composition of plants
Chemical composition of plant can be analyse by following way.
1. Fresh weight:
1. Fresh weight:
- Uproot a plant and wash down the soil particles sticking to the roots.Weigh the entire plant. This weight is called fresh weight of the plant.
- Dry this plant in oven at 100-110 degree celsius to remove the water that forms the major constituent of the plant body.
- Weight of this dried plant from which all the water has been dried off is called the dry weight of the plant
- When an oven-dried plant material is ignited at 400-600 degree C, all the organic materials are oxidized and incombustible matter remains as plant ash.
- When this ash was analysed it was found to contain 40 elements besides C, H, O, N and S which were oxidized.
- All these are not essential for plant nutrition but on analysis the important essential elements have been identified and based on their role in plant metabolism and requirement, they have been classified as major elements and trace elements.
Classification of mineral nutrients
Following criterion can be used to classify the mineral nutrients:
- Metabolic need for the mineral nutrient (Essential and non essential)
- Amount required or present in plant tissue (Macro and micro nutrients)
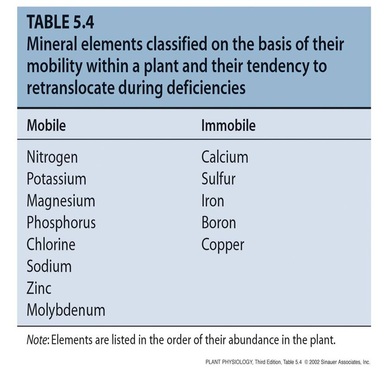
- Mobility within the plant (Mobile and immobile)
- Biochemical function(s) for the mineral nutrient
1. Essential and non-essential mineral elements
|
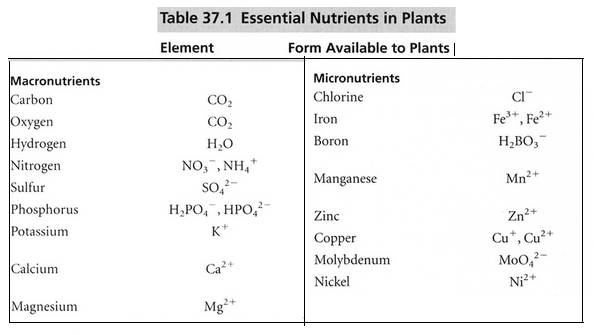
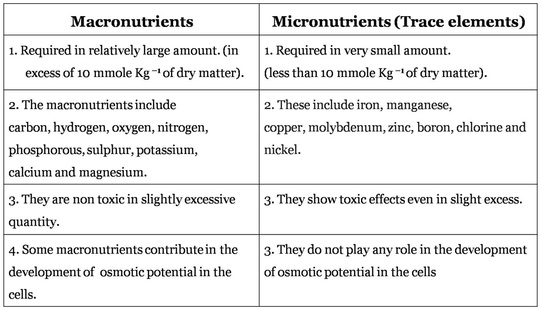
2. Macronutrients and micronutrients
3. Mobile and immobile mineral nutrients
4.Role of minerals
- Components of plant body and protoplast.
- Catalytic compounds.
- Osmotic potential.
- Acidity and buffer action
- Maintenance of electrostatic neutrally
Sources of essential elements for plants
|
Soil-less culture or solution culture
|
Hydroponics
|
Advantages:
| Disadvantages:
|
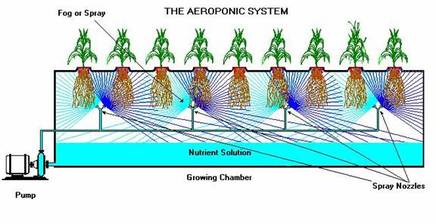
Aeroponics
Sand culture
|
|
|
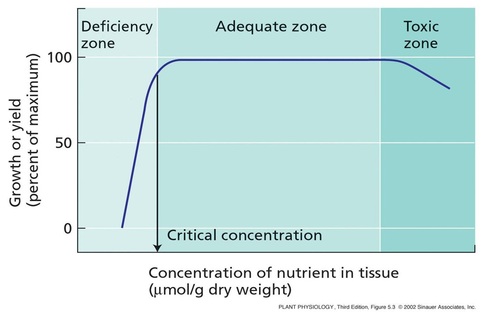
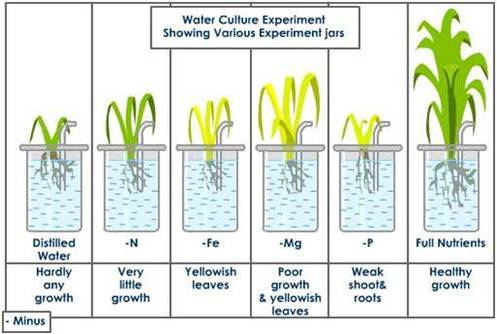
Deficiency symptoms of mineral nutrients
- Deficiency of the mineral results into certain morphological abnormalities.
- Any visible deviation from the normal structure and function of the plant is called a symptom.
- Stunting: The growth is retarded. The Stem appears condensed and short.
- Chlorosis: It is the loss of chlorophyll resulting in the yellowing of leaves.
- Necrosis: Localized death of tissues of leaves.
- Mottling: Appearances of patches of green and non green on the leaves.
- Leaf curles: Abnormal curling of leaves due to unequal growth.
- Abscission: Premature fall of flowers, fruits and leaves.
- Wilting: Loss of turgor in the cells resulting in the drooping of leaves and young stem and tips.
- Heart rot:Internal softening and rottening of tissue due to their disintegration.
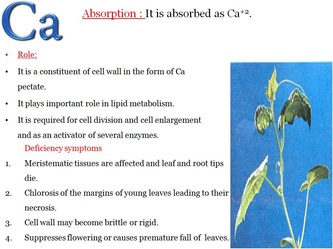
Role and deficiency symptoms of Macronutrients
Role and deficiency symptoms of Micronutrients (Given in PPT )
UPTAKE OF MINERALS :
MECHANISM :
|
THEORIES
Several theories have been put forth to explain the mechanism of translocation of mineral salts. These theories can be placed under two headings
(i) Passive absorption and
(ii) Active Absorption
I) Passive Absorption
Several theories have been put forth to explain the mechanism of translocation of mineral salts. These theories can be placed under two headings
(i) Passive absorption and
(ii) Active Absorption
I) Passive Absorption
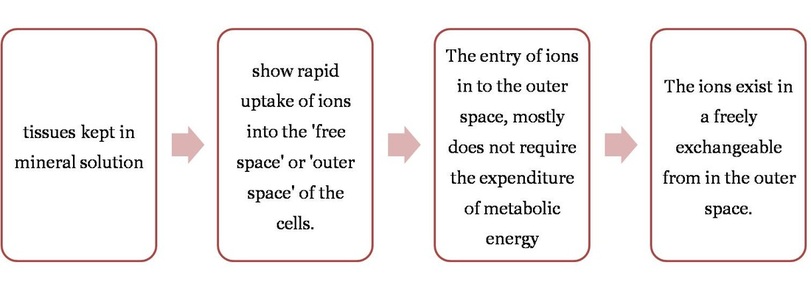
- When the movement of mineral ions into the roots occurs by diffusion without any expenditure of energy in the form of ATP it is called Passive Absorption.
- This form of absorption is not affected by temperature and metabolic inhibitors.
- Rapid uptake of ions is observed when a plant tissue in transferred from a medium of low concentration to a medium high concentration.
- Various theories have been put forward to explain mineral salt uptake by passive absorption.
- Ion-exchange theory
- Mass flow
- Donnan equilibrium
1. Ion exchange theory:
Mineral elements are absorbed in the form of ions. The anions and cations within the plant cells are exchanged with the anions and cations of equivalent
charge from the external medium in which the cells are kept. This mechanism can be explained by two theories.
Mineral elements are absorbed in the form of ions. The anions and cations within the plant cells are exchanged with the anions and cations of equivalent
charge from the external medium in which the cells are kept. This mechanism can be explained by two theories.
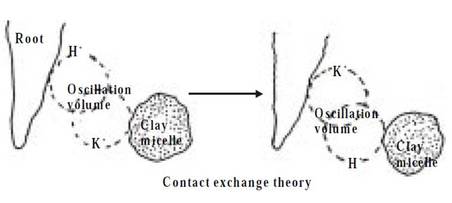
A) Contact exchange theory
|
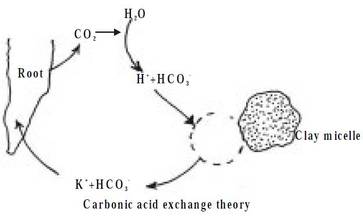
B) Carbonic acid exchange theory
|
II. Active absorption
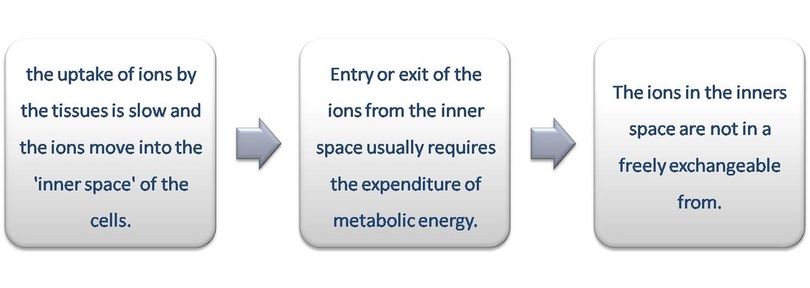
The absorption of ions against the concentration gradient with the expenditure of metabolic energy is called active absorption. In plants, the vacuolar sap shows accumulation of anions and cations against the concentration gradient which cannot be explained by the theories of passive absorption. The mechanism of active absorption of salts can be explained by several theories.
1. Carrier concept
2. Cytochrome Pump Theory or Electron Transport Theory
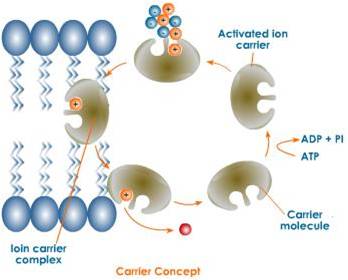
1. Carrier concept:
The absorption of ions against the concentration gradient with the expenditure of metabolic energy is called active absorption. In plants, the vacuolar sap shows accumulation of anions and cations against the concentration gradient which cannot be explained by the theories of passive absorption. The mechanism of active absorption of salts can be explained by several theories.
1. Carrier concept
2. Cytochrome Pump Theory or Electron Transport Theory
1. Carrier concept:
- The cell shows the presence of carriers or transporters which are highly specific for a particular ion.
- The carrier picks up an ion from external medium to form a carrier-ion complex, undergoes rotation at 180 degree, moves across the membrane and releases the ions on the inner side of membrane and returns to pick up another ion.
- The carrier may be an enzyme or a protein. Metabolic energy (ATP) is expended in this process.
NITROGEN FIXATION
- Nitrogen is an inert gas which constitutes 78% of the atmosphere.
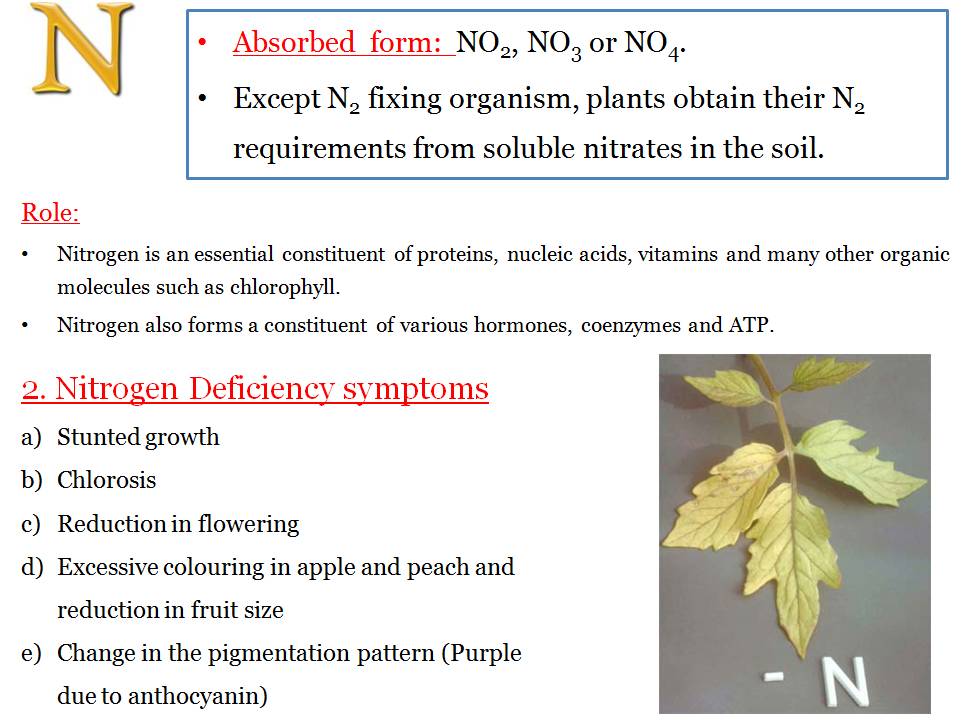
- It is an important mineral present in the bodies of living organisms. It forms a component of proteins and amino acids and is also present in nucleic acids, cytochromes, chlorophyll, vitamins, alkaloids and so on.
- Nitrogen cannot be used directly and is converted to Nitrites, Nitrates and Ammonia by a process called Nitrogen Fixation.
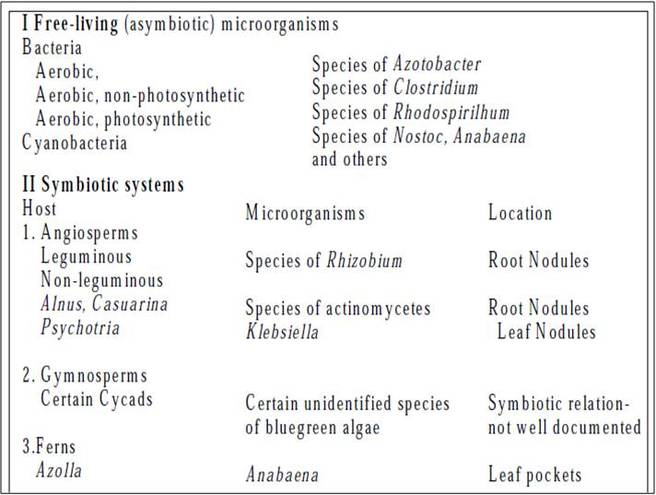
- There are many free living organisms like bacteria and blue-green algae which are involved in nitrogen fixation.
- The ammonia and urea present in the soil are directly absorbed by plants.
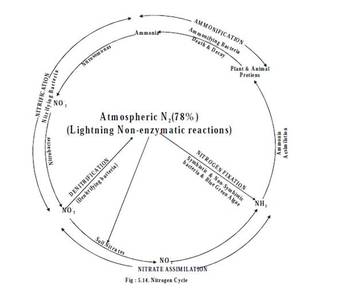
Nitrogen cycle
- The atmosphere is the source of elemental nitrogen which cannot be used directly by plants. The atmospheric nitrogen is converted to ammonia, nitrite, nitrate or organic nitrogen in the soil.
- The death and decay of organic systems causes cycling of ammonia from amino acids, purines and pyrimidines. Some of these forms may also be converted to Nitrogen gas and may be cycled back into the atmosphere.
- The process by which these forms get inter converted to maintain a constant amount of nitrogen in atmosphere, by physical and biological processes is called nitrogen cycle. The cycle includes 5 stages.
- Ammonification
- Nitrification
- Nitrate assimilation
- Denitrification and
- Nitrogen fixation
(i) Ammonification
(ii) Nitrification
- Conversion of organic nitrogen to ammonium ions by microbes present in the soil is called ammonification.
- The sources of organic nitrogen in the soil are animal excreta and dead and decaying plant and animal remains which are acted upon by ammonifying saprotrophic bacteria such as Bacillus ramosus, Bacillus vulgaris, certain soil fungi and actinomycetes.
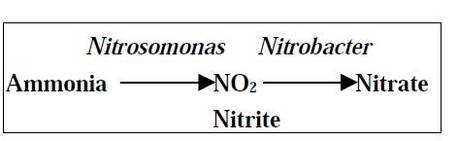
(ii) Nitrification
- Conversion of ammonia to nitrite (NO2 -) and then nitrate (NO3 -) is known as nitrification.
- Nitrifying bacteria like Nitrosomonas convert ammonia to nitrite and another bacterium called Nitrobacter converts nitrate to nitrate.
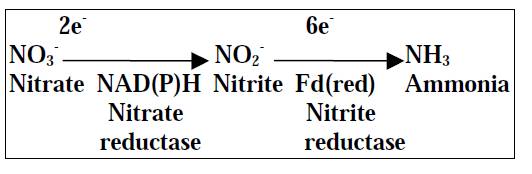
(iii) Nitrate Assimilation
- The nitrate present in the soil is absorbed by plants through the root system in the form of NO3 - ions. But it cannot be used by plants directly. So it is first reduced to nitrite by the enzyme nitrate reductase.
- Nitrite is then converted to Ammonia by the enzyme nitrite reductase. This reduction of Nitrate to Ammonia and its incorporation into cellular proteins by aerobic microorganisms and higher plants is called Nitrate assimilation.
(iv) Dentrification
- The process of conversion of nitrate and nitrite into ammonia, nitrogen gas and nitrous oxide (N2O) is called denitrification.
- This process ends in the release of gaseous nitrogen into the atmosphere and thus completes the nitrogen cycle.
- A number of bacteria such as Pseudomonas denitrificans, Bacillus subtilis and Thiobacillus dentrificans are involved in this process.
(v) Nitrogen fixation
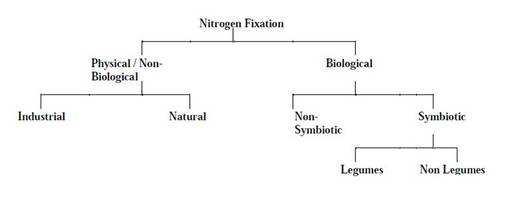
- Nitrogen fixation refers to the conversion of elementary dinitrogen into organic form to make it available for plants.
Nitrogen fixation
Nitrogen fixation is of two types-
|
These organisms may be freeliving which are otherwise called non-symbiotic nitrogen fixers and may form symbiotic associations when they are called
symbiotic nitrogen fixers.
symbiotic nitrogen fixers.
Nitrogen fixation in legumes
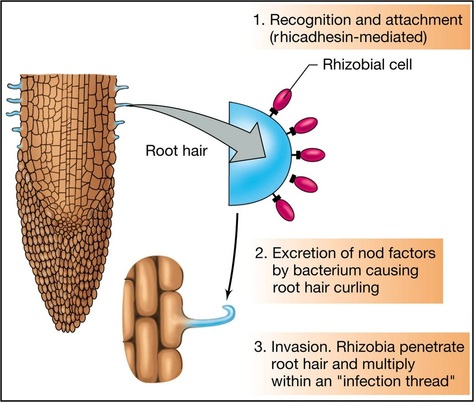
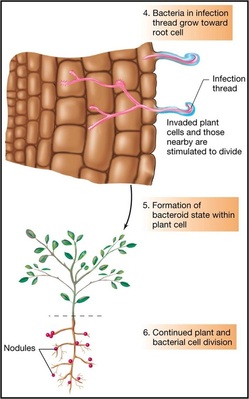
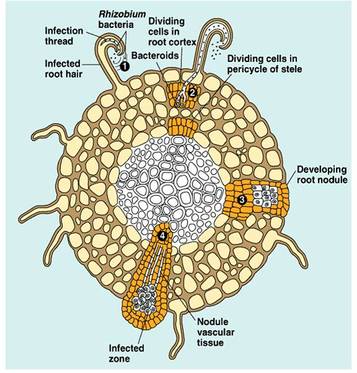
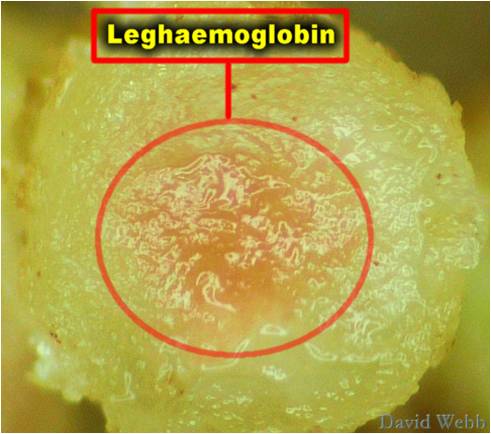
This is the commonest type of symbiotic nitrogen fixation which has been elaborately studied. A soil bacterium called Rhizobium infects roots of leguminous plants (belonging to Family Leguminosae) and forms the root nodules. These are involved in nitrogen fixation. The bacteria living in the soil enter the root hair and penetrate the root cortex through an infection thread. When the bacteria enter the cortical cells of roots, the latter get stimulated to divide vigorously and form nodules on the root. The bacteria come to occupy the nodules, and at this stage lack a rigid cell wall being called bacteroids. These make use of the food substances of the root cells and secrete a pinkish pigment called leg-hemoglobinwhich is an oxygen carrier like hemoglobin.
The Rhizobia in the form of bacteroides contain the enzyme nitrogenase which is responsible for fixation of Nitrogen thus benefitting the host plant.Leghemoglobin is supposed to protect the nitrogenase enzyme as it can functiononly under anaerobic conditions.
The Rhizobia in the form of bacteroides contain the enzyme nitrogenase which is responsible for fixation of Nitrogen thus benefitting the host plant.Leghemoglobin is supposed to protect the nitrogenase enzyme as it can functiononly under anaerobic conditions.
The process can be summarize in following points.
![]()
![]()
![]()
- Chemical recognition between roots and Rhizobium
- Root hair curling.
- Formation of infection thread.
- Invasion of roots by Rhizobia
- Cortical cell divisions and formation of nodule tissue
- Bacteria fix nitrogen which is transferred to plant cells in exchange for fixed carbon.