What are other names or identifying information for chlorine?
CAS Registry No.: 7782-50-5
Other Names: Liquefied chlorine gas, Chlorine gas
Main Uses: Manufacture of other chemicals, bleaching agent, water purification.
Appearance: Green - yellow gas. Clear yellow or amber liquid (under pressure).
Odour: Pungent
Canadian TDG: UN1017
Other Names: Liquefied chlorine gas, Chlorine gas
Main Uses: Manufacture of other chemicals, bleaching agent, water purification.
Appearance: Green - yellow gas. Clear yellow or amber liquid (under pressure).
Odour: Pungent
Canadian TDG: UN1017
What is the WHMIS 1988 classification?
A - Compressed Gas; D1A - Very Toxic; E - Corrosive
![Symbol for Class A]()
Class A![Symbol for Class D1]()
Class D1A![Symbol for Class E]()
Class E
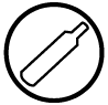
Class A

Class D1A
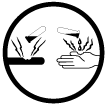
Class E
What are the most important things to know about chlorine in an emergency?
Emergency Overview: Green - yellow gas. Pungent odour. Will not burn. COMPRESSED GAS. Contains gas under pressure. May explode if heated. OXIDIZER. May cause or intensify fire. Highly Reactive. Incompatible with many common chemicals. VERY TOXIC. Fatal if inhaled. Corrosive to the respiratory tract. A severe, short-term exposure may cause long-term respiratory effects (e.g., Reactive Airways Dysfunction (RADS)). CORROSIVE. Causes severe skin burns and eye damage. May cause frostbite.
What are the most important things to know about chlorine in an emergency?
Emergency Overview: Inhalation. Skin contact. Eye contact.
- Inhalation: VERY TOXIC, can cause death. Can cause severe irritation of the nose and throat. Can cause severe lung injury. Can cause life-threatening accumulation of fluid in the lungs (pulmonary edema). Symptoms may include coughing, shortness of breath, difficult breathing and tightness in the chest. Symptoms may develop hours after exposure and are made worse by physical effort. Long-term damage may result from a severe short-term exposure. A single exposure to a high concentration can cause a long-lasting condition like asthma. If this occurs, many things like other chemicals or cold temperatures can easily irritate the airways. Symptoms may include shortness of breath, tightness in the chest and wheezing. {Reactive Airways Dysfunction Syndrome (RADS)}.
- Skin Contact: CORROSIVE. The gas irritates or burns the skin. Permanent scarring can result. Direct contact with the liquefied gas can chill or freeze the skin (frostbite). Symptoms of mild frostbite include numbness, prickling and itching. Symptoms of more severe frostbite include a burning sensation and stiffness. The skin may become waxy white or yellow. Blistering, tissue death and infection may develop in severe cases.
- Eye Contact: CORROSIVE. The gas irritates or burns the eyes. Permanent damage including blindness can result. Direct contact with the liquefied gas can freeze the eye. Permanent eye damage or blindness can result.
- Ingestion: Not a relevant route of exposure (gas).
- Effects of Long-Term (Chronic) Exposure: May harm the respiratory system. Can irritate and inflame the airways.
- Carcinogenicity: Not known to cause cancer.
International Agency for Research on Cancer (IARC): Not specifically evaluated.
American Conference for Governmental Industrial Hygienists (ACGIH): A4 - Not classifiable as a human carcinogen.
- Teratogenicity / Embryotoxicity: Not known to harm the unborn child.
- Reproductive Toxicity: Not known to be a reproductive hazard.
- Mutagenicity: Not known to be a mutagen.
What are first aid measures for chlorine?
Inhalation: Take precautions to ensure your own safety before attempting rescue (e.g. wear appropriate protective equipment). Move victim to fresh air. Keep at rest in a position comfortable for breathing. If breathing is difficult, trained personnel should administer emergency oxygen. DO NOT allow victim to move about unnecessarily. Symptoms of pulmonary edema may be delayed. Immediately call a Poison Centre or doctor. Treatment is urgently required. Transport to a hospital.
Skin Contact: Gas: Flush with lukewarm, gently flowing water for 5 minutes. If irritation or pain persists, see a doctor. Liquefied gas: quickly remove victim from source of contamination. DO NOT attempt to rewarm the affected area on site. DO NOT rub area or apply direct heat. Gently remove clothing or jewelry that may restrict circulation. Carefully cut around clothing that sticks to the skin and remove the rest of the garment. Loosely cover the affected area with a sterile dressing. DO NOT allow victim to drink alcohol or smoke. Immediately call a Poison Centre or doctor. Treatment is urgently required. Transport to a hospital.
Eye Contact: Gas: immediately flush the contaminated eye(s) with lukewarm, gently flowing water for 5 minutes, while holding the eyelid(s) open. If irritation or pain persists, see a doctor. Liquefied gas: avoid direct contact. Wear chemical protective gloves if necessary. Immediately and briefly flush with lukewarm, gently flowing water. DO NOT attempt to rewarm. Cover both eyes with a sterile dressing. DO NOT allow victim to drink alcohol or smoke. Immediately call a Poison Centre or doctor. Treatment is urgently required. Transport to a hospital.
Ingestion: Not applicable (gas).
First Aid Comments: All first aid procedures should be periodically reviewed by a doctor familiar with the chemical and its conditions of use in the workplace.
Skin Contact: Gas: Flush with lukewarm, gently flowing water for 5 minutes. If irritation or pain persists, see a doctor. Liquefied gas: quickly remove victim from source of contamination. DO NOT attempt to rewarm the affected area on site. DO NOT rub area or apply direct heat. Gently remove clothing or jewelry that may restrict circulation. Carefully cut around clothing that sticks to the skin and remove the rest of the garment. Loosely cover the affected area with a sterile dressing. DO NOT allow victim to drink alcohol or smoke. Immediately call a Poison Centre or doctor. Treatment is urgently required. Transport to a hospital.
Eye Contact: Gas: immediately flush the contaminated eye(s) with lukewarm, gently flowing water for 5 minutes, while holding the eyelid(s) open. If irritation or pain persists, see a doctor. Liquefied gas: avoid direct contact. Wear chemical protective gloves if necessary. Immediately and briefly flush with lukewarm, gently flowing water. DO NOT attempt to rewarm. Cover both eyes with a sterile dressing. DO NOT allow victim to drink alcohol or smoke. Immediately call a Poison Centre or doctor. Treatment is urgently required. Transport to a hospital.
Ingestion: Not applicable (gas).
First Aid Comments: All first aid procedures should be periodically reviewed by a doctor familiar with the chemical and its conditions of use in the workplace.
What are first aid measures for chlorine?
Inhalation:Does not burn. Strong OXIDIZER. May intensify fire.
Suitable Extinguishing Media: Not combustible. Use extinguishing agent suitable for surrounding fire.
Specific Hazards Arising from the Chemical: Heat from fire can cause a rapid build-up of pressure inside cylinders. Explosive rupture and a sudden release of large amounts of gas may result. Cylinder may rocket. In a fire, the following hazardous materials may be generated: corrosive hydrogen chloride.
Suitable Extinguishing Media: Not combustible. Use extinguishing agent suitable for surrounding fire.
Specific Hazards Arising from the Chemical: Heat from fire can cause a rapid build-up of pressure inside cylinders. Explosive rupture and a sudden release of large amounts of gas may result. Cylinder may rocket. In a fire, the following hazardous materials may be generated: corrosive hydrogen chloride.
What are the stability and reactivity hazards of chlorine?
- Chemical Stability: Normally stable.
- Conditions to Avoid: High temperatures. Temperatures above 52°C.
- Incompatible Materials: Highly reactive. Reacts explosively with: many chemicals, including, alcohols (e.g. ethanol), ammonia, saturated hydrocarbons (e.g. butane), aldehydes (e.g. acetaldehyde), metals (e.g. aluminum), ethers (e.g. diethyl ether). Corrosive to: aluminum alloys, carbon steel, and other metals.
- Hazardous Decomposition Products: Corrosive hydrogen chloride.
- Possibility of Hazardous Reactions: Strong OXIDIZER. May cause or intensify fire.
What are accidental release measures for chlorine?
Personal Precautions: Evacuate the area immediately. Isolate the hazard area. Keep out unnecessary and unprotected personnel. Vapour or gas may accumulate in hazardous amounts in low-lying areas especially inside confined spaces, if ventilation is not sufficient. Remove or isolate incompatible materials as well as other hazardous materials.
Methods for Containment and Clean-up: Small spills or leaks: stop or reduce leak if safe to do so. Ventilate the area to prevent the gas from accumulating, especially in confined spaces. Large spills or leaks: stop or reduce leak if safe to do so. Ventilate the area to prevent the gas from accumulating, especially in confined spaces. If possible, turn leaking container so that gas escapes rather than liquefied gas. Knock down gas with fog or fine water spray. Do not direct water at spill or source.
Other Information: Contact supplier, local fire and emergency services for help.
Methods for Containment and Clean-up: Small spills or leaks: stop or reduce leak if safe to do so. Ventilate the area to prevent the gas from accumulating, especially in confined spaces. Large spills or leaks: stop or reduce leak if safe to do so. Ventilate the area to prevent the gas from accumulating, especially in confined spaces. If possible, turn leaking container so that gas escapes rather than liquefied gas. Knock down gas with fog or fine water spray. Do not direct water at spill or source.
Other Information: Contact supplier, local fire and emergency services for help.
What handling and storage practices should be used when working with chlorine?
Handling: In event of a spill or leak, immediately put on escape-type respirator and exit the area. Immediately report leaks, spills or failures of the safety equipment (e.g. ventilation system). Secure cylinder in an up-right position. Protect cylinders from damage. Use a suitable hand truck to move cylinders; do not drag, roll, slide, or drop. Use the pressure regulator appropriate for cylinder pressure and contents.
Storage: Store in an area that is: cool, dry, well-ventilated, out of direct sunlight and away from heat and ignition sources, secure and separate from work areas, separate from incompatible materials, on the ground floor or preferably, in an isolated, detached building. Always secure (e.g. chain) cylinders in an upright position to a wall, rack or other solid structure. Label container with date received, date opened and disposal date. Use a first-in, first-out inventory system. Empty containers may contain hazardous residue. Store separately. Keep closed. Comply with all applicable health and safety regulations, fire and building codes.
Storage: Store in an area that is: cool, dry, well-ventilated, out of direct sunlight and away from heat and ignition sources, secure and separate from work areas, separate from incompatible materials, on the ground floor or preferably, in an isolated, detached building. Always secure (e.g. chain) cylinders in an upright position to a wall, rack or other solid structure. Label container with date received, date opened and disposal date. Use a first-in, first-out inventory system. Empty containers may contain hazardous residue. Store separately. Keep closed. Comply with all applicable health and safety regulations, fire and building codes.
What is the American Conference of Governmental Industrial Hygienists (ACGIH®) recommended exposure limit for chlorine?
ACGIH® TLV® - TWA : 0.5 ppm A4
ACGIH® TLV® - STEL [C]: 1 ppm
Exposure Guideline Comments: TLV® = Threshold Limit Value. TWA = Time-Weighted Average. STEL = Short-term exposure Limit. C = Ceiling limit. A4 = Not classifiable as a human carcinogen.
ACGIH® TLV® - STEL [C]: 1 ppm
Exposure Guideline Comments: TLV® = Threshold Limit Value. TWA = Time-Weighted Average. STEL = Short-term exposure Limit. C = Ceiling limit. A4 = Not classifiable as a human carcinogen.
What are the engineering controls for chlorine?
Engineering Controls: Use a local exhaust ventilation and enclosure, if necessary, to control amount in the air. Consider using Use a corrosion-resistant exhaust ventilation system separate from other ventilation systems. It may be necessary to use stringent control measures such as process enclosure to prevent product release into the workplace. Use backup controls (e.g. double mechanical pump seals) to prevent the release of this material due to equipment failure. Provide eyewash and safety shower if contact or splash hazard exists.
What Personal Protective Equipment (PPE) is needed when working with chlorine?
Eye/Face Protection: Wear chemical safety goggles. A face shield (with safety goggles) may also be necessary.
Skin Protection: Wear chemical protective clothing e.g. gloves, aprons, boots. Coveralls or long sleeve shirts and pants in some operations. Wear a chemical protective, full-body encapsulating suit and self-contained breathing apparatus (SCBA). Suitable materials include: butyl rubber, neoprene rubber, Viton®, Viton®/butyl rubber, Barrier® (PE/PA/PE), Silver Shield/4H® (PE/EVAL/PE), Trellchem® HPS, Trellchem® VPS, Tychem® SL (Saranex™), Tychem® BR/LV, Tychem® Responder, Tychem® TK. The following materials should NOT be used: polyethylene, polyvinyl chloride. Recommendations are NOT valid for very thin Neoprene and Nitrile gloves (0.3 mm or less).
Respiratory Protection: Up to 5 ppm: wear a NIOSH approved air-purifying respirator with an appropriate cartridge, or supplied air respirator. Up to 10 ppm: wear a powered air-purifying respirator with an appropriate cartridge, wear a full facepiece NIOSH approved air-purifying respirator with an appropriate cartridge, wear a NIOSH approved self-contained breathing apparatus (SCBA) or supplied air respirator. For non-routine or emergency situations: wear a NIOSH approved air-purifying respirator with an appropriate cartridge, wear a NIOSH approved self-contained breathing apparatus (SCBA) or supplied air respirator. Eye protection may be required.
Skin Protection: Wear chemical protective clothing e.g. gloves, aprons, boots. Coveralls or long sleeve shirts and pants in some operations. Wear a chemical protective, full-body encapsulating suit and self-contained breathing apparatus (SCBA). Suitable materials include: butyl rubber, neoprene rubber, Viton®, Viton®/butyl rubber, Barrier® (PE/PA/PE), Silver Shield/4H® (PE/EVAL/PE), Trellchem® HPS, Trellchem® VPS, Tychem® SL (Saranex™), Tychem® BR/LV, Tychem® Responder, Tychem® TK. The following materials should NOT be used: polyethylene, polyvinyl chloride. Recommendations are NOT valid for very thin Neoprene and Nitrile gloves (0.3 mm or less).
Respiratory Protection: Up to 5 ppm: wear a NIOSH approved air-purifying respirator with an appropriate cartridge, or supplied air respirator. Up to 10 ppm: wear a powered air-purifying respirator with an appropriate cartridge, wear a full facepiece NIOSH approved air-purifying respirator with an appropriate cartridge, wear a NIOSH approved self-contained breathing apparatus (SCBA) or supplied air respirator. For non-routine or emergency situations: wear a NIOSH approved air-purifying respirator with an appropriate cartridge, wear a NIOSH approved self-contained breathing apparatus (SCBA) or supplied air respirator. Eye protection may be required.