Oxygen
Oxygen - A Typical Nonionizing Gas
Dissolved oxygen in water is a major factor affecting water system operations and water treatment programs. Oxygen causes corrosion, and at the same time assists in forming passive, protective films on metal surfaces. Some water treatment programs require dissolved oxygen for good performance, while others work best in the absence of oxygen.
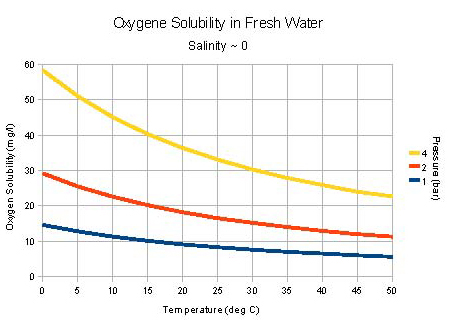
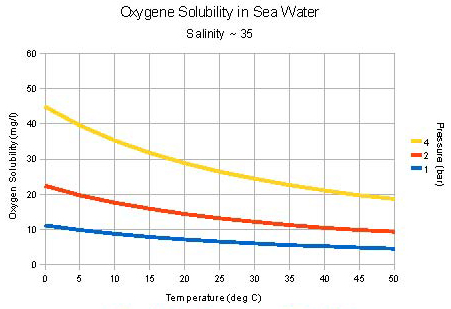
Figure 1-7 shows the solubility of oxygen in water as a function of temperature and pressure. At 0 °C and atmospheric pressure, the solubility is about 13 mg/L. At 25 °C it is 8 mg/L, and in boiling water the solubility is zero. Most natural water supplies contain dissolved oxygen. Rain and surface waters are usually saturated, while well waters may be low in oxygen due to chemical and microbiological reactions.