Empirical fit of saturated vapor density versus Celsius Temperature
![]()
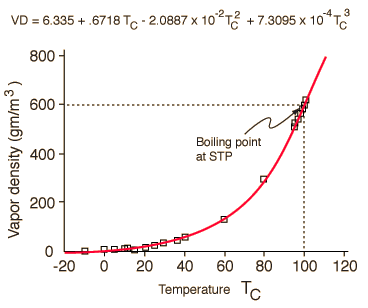
It is possible to produce what appears to be a good fit of the saturated vapor density of water all the way up to the boiling point. But for the purposes of calculating relative humidity, the values near boiling are not important and are given too much emphasis in the empirical fit above. The behavior of water vapor density is a non-linear function, but an approximate calculation of saturated vapor density can be made from an empirical fit of the vapor density curve
![]()
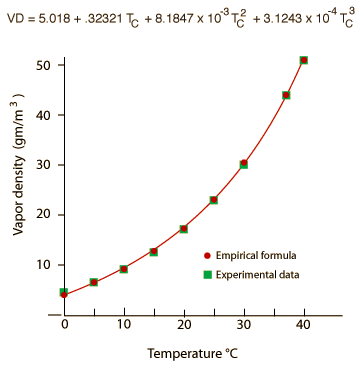
If only the values up to 40°C are used for the fit, a more precise fit of the data is obtained in the temperature region where relative humidity is of interest. This is the fit used in the calculation of relative humidity below, but it significantly underestimates the vapor density near the boiling point.
The saturated vapor pressure reaches 760 mmHg at 100°C, the standard boiling point. The saturated vapor pressure roughly parallels the saturated vapor density; numerical values are included in the vapor density table.