Air Pollution: Essay on Air Pollution
Air is the most vital constituent of environment for the sustenance of life on earth. Air forms nearly 80% of man’s daily intake by weight. In pure air, the proportion of different constituents like oxygen, nitrogen and other gases is fixed and definite.
It may be noted that air cannot be pure because some gases like sulphur dioxide, carbon monoxide, oxides of nitrogen, emission from volcanoes and swamps, salt spray, pollens from plants etc., are continuously added to the air by natural processes. Thus, air is polluted when its natural composition is disturbed either by natural or by man-made sources.
Contents:
1. Classification of Air Pollutants
2. Source of Air Pollution
3. Important Air Pollutants
4. Effects of Air Pollution
5. Consequences of Air Pollution
6. Control of Air Pollution
H. Perkins (1974) has defined air pollution as, “the presence in the outdoor atmosphere of one or more contaminants such as dust, fumes, gases, mist, smoke or vapours in quantities of characteristics and of duration such as to be injurious to living organisms and to property which reasonably interferes with comfortable enjoyment of life and property.
According to World Health Organization (WHO), air pollution is defined as limited to situation in which the outdoor ambient atmosphere contains materials in concentration which are harmful to man and its environment. In general sense, air pollution may be defined as the imbalance or disequilibrium in the quality of air due to introduction of foreign materials from natural or anthropogenic sources to the air so as to cause adverse effect to biological communities in general and man in particular.
The nature, dimension and magnitude of air-pollution depend upon a number of factors such as source of pollutants, nature of pollutants, quantity of pollutants, residence time of the pollutants in atmosphere. According to H.E. Hobbs (1980), the residence time of pollutants depend upon nature of the pollutants, their way of emission, meteorological factors and on sink mechanisms. Mainly there are two factors which contribute to the problem of air pollution. These are population explosion and productivity, each rising 2 to 3 per cent every year.
1. Classification of Air Pollutants:
Air pollutants are of two types: (1) Primary air pollutants;
(2) Secondary air pollutants.
1. Primary air pollutants:
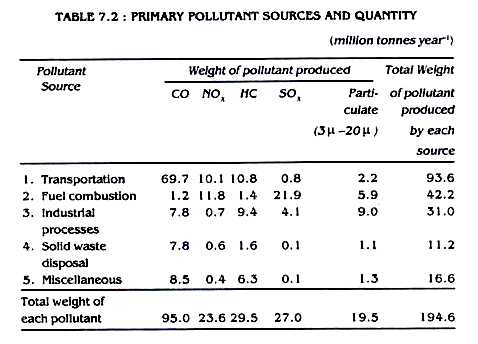
These are the harmful chemicals which directly enter into air due to natural events or human activities. For example, when carbon containing substance is burnt, it will release CO2 and/or CO to atmosphere. These gases directly entering into atmosphere influence the biosphere are termed as primary pollutants.It is seen that there are mainly five primary pollutants which contribute together more than 90% of global air pollution. These are namely, carbon monoxide (CO) nitrogen oxides (NO2), oxides of sulphur (SO2) hydrocarbons (HC), and particulates.
Transportation accounts for more than 46% of the total pollutants produced per year and hence remains as the principal source of air pollution. Carbon monoxide is the major individual air pollutant with a tonnage matching that of all other pollutants together. Different primary pollutants, their sources and amounts, released to air are given in table 7.2.
2. Secondary Air-Pollutants:
These are the harmful chemicals produced in air due to chemical reaction in between two or more components i.e. the reaction in between primary air pollutant and some components of air. Usually the primary air pollutant suffers chemical changes in presence of H2O (v), O2 (g) and ultraviolet radiation of sun to form secondary pollutants.Some reactions showing conversion of primary pollutant to secondary pollutant are described below:
Primary pollutant Reactant Secondary Pollution
| 1. | CO2 | + | H2O | →H2CO3 (Carbonic acid) |
| 2. | H2S | + | 2O2 | →H2SO4 (Sulphuric acid) |
| 3. | SO2 | + | O2 | →SO3, |
| SO3 | + | H2O | →H2SO4 (Sulphuric acid) | |
| 4. | NO2 | + | O2 | →2N2Os |
| + | H2O | →2HNO3 (Nitric acid) | ||
| 5. | HF (g) | + | H2O | →HF (aq) |
| 6. | SiF6 | + | 2HF | →H2SiF6 |
2. Source of Air Pollution:
The Principal sources of air pollution can either be natural or anthropogenic. (a) Natural Sources:
The natural sources of air pollution are:
(i) Forest Fire,
(ii) Wind,
(iii) Soil Erosion,
(iv)Volcanic Eruption,
(v) Evaporation of volatile organic matter, and
(vi) Bacterial decomposition products.
(b) Anthropogenic Sources:
(i) Automobile Exhaust:
These contain carbon monoxide (CO), oxides of nitrogen, ethane, ethylene, 3, 4- benzpyrine. These gases are due to incomplete combustion of petrol and diesel.
(ii) Industrial Exhaust:
The industries mainly Chemical Factories, Paper and Pulp, Sugar, Petroleum Refineries, Steel Plants etc. are the chief air polluting agents. The industrial exhaust contain gases like CO, CO2, SO2, NO,
NO2, N2O, Cl2, F2, NH3, and particulate matters.
(iii) Release of organic substances : The processes like biological decomposition of organic matter, seepage from natural gas and oil fields, volatile emission from plants, sewage gas etc. are the causes for the release of organic substances like CH4, C2H6, C6H5NH2, C2H4 etc.
(iv) Release of Chlorofluorocarbons (CFCs):
The chlorofluorocarbons are released to the atmosphere from air conditioners, refineries, pre cooler systems of cold storage etc.
(v) Photochemical oxidants:
These are ozone and peroxyacylnitrate (PAN) formed by certain photochemical reactions.
(vi) Tobacco smokes; it is produced by cigarette and bidis.
(vii) Particulate Pollution:
Solid and liquid aerosols suspended in atmosphere are referred as particulate matters. These arise from grinding of ores, spraying and soil erosion. Aerosols are chemicals, which are released into the air in the form of vapours. For example lead containing gasoline fumes from automobiles, constitute the chief source of lead contamination.
(viii) Agricultural Chemicals:
The agricultural chemicals like pesticides, insecticides, fungicides, herbicides etc. are also released into the air as pollutants.
(ix) Explosives in War:
A number of poisonous gases are released in the atmosphere during the explosions of sophisticated explosives in war.
(x) Photochemical Smogs:
When different gases like SO2, NO, N2O, NO2 and un-burnt hydrocarbons are released into atmosphere, these form photochemical smog by combining with dust and moisture.
(xi) Mining Activity:
During mining activities i.e. crushing and grinding of ores, a lot of particulate matters are released to atmosphere.
(xii) Release from fertiliser plants:
Ammonia gas is released from ammonium fertilizer plants

(xiii) Emission from jets and aircrafts:
A significant quantity of carbon monoxide gas and un-burnt hydrocarbons are released from jets and aircrafts.
(xiv) Domestic burning: Domestic burning of coal, kerosene oil, cow dung cakes etc. releases CO2, CO, SO2, etc.
3. Important Air Pollutants:
Pollutants are the various harmful chemicals present in the atmosphere in concentrations that disturb the dynamic equilibrium in the atmosphere and thereby affects the living organisms and their environment. These are released to the environment partly or completely by anthropogenic activities or natural processes. Some potent chemical pollutants, their sources, mechanism of action in environment, detrimental effects and their control measures are described below. 1. Carbon monoxide (Co):
A. Source:Natural process like volcanic action, electrical discharge during storm or lightening, seed germination, marsh gas production etc. release small quantity of Carbon monoxide to the atmosphere. The significant contribution of carbon monoxide is from anthropogenic activities. For example out of the annual emission of 350 million tonnes, human activities contribute 275 million tonnes and natural processes contribute only 75 million tonnes. Some important CO producing processes are:
(i) Transportation:
It contributes about 74% of CO through motor vehicles aircrafts, rail, roads etc.
(ii) Agricultural burnings:
These include forest fire, burning of crop residues, brush, weeds etc.
(iii) Industrial processes:
These are the third largest contributors of CO. Industries like iron, steel, paper, petroleum etc. release large quantity of CO to the atmosphere.
B. Sink:
The release of CO to the atmosphere is taking place in such a large scale that the concentration is supposed to be doubled in every five years. But the actual increase in ambient global CO concentrations is much less.
The lowering of the concentration of CO is atmosphere may be due to:
(i) The oxidation of CO to CO2 in the atmosphere by atomic oxygen, hydroxyl radical, NO2, singlet O2 etc.
(ii) Some micro-organisms present in the soil help in the oxidation of CO to CO2.
(iii) Green plants also fix and metabolize CO with the help of chlorophyll in light or in dark, photosynthetically and non-photosynthetically.
C. Detrimental Effects:
(a) On Human beings:
CO interferes with oxygen carrying function of hemoglobin by forming carboxy hemoglobin complex.
(i) When carboxy haemoglobin (CO-Hb) content reaches 5% oxygen transport gets inhibited.
(ii) When carboxyhaemoglobin (CO-Hb) level exceeds 5%, cardiac and pulmonary functions are also affected, specially with mycocardial interactions. The poisonous effect of CO is due to the fact that it prevents the R.B.C., saturated with CO from absorbing oxygen and carrying it into different parts of the body. Death is caused by asphyxiation.
(iii) When CO concentration becomes 400-500 ppm, it leads to loss of fertility, premature birth, spontaneous abortion and deformed babies in pregnant women.
(b) On Plants:
Plants are insensitive to basal level of CO. However, prolonged exposure of plants to higher concentrations of CO causes hazards in plants.
(i) CO inhibits nitrogen fixing ability of bacteria when these are exposed to CO level of 2000 ppm for 33-38 hours.
(ii) CO level within 100 to 10000 ppm causes lead drop, premature aging, leaf curling, reduction of leaf size, etc.
(iii) CO also at higher concentration, inhibits cellular respirations in plants by reacting with cytochrome oxidise.
D. Control of Co-Emission:
Extensive investigations suggest that about 74% of all the CO emission is from automobiles. So control efforts must be focused on automobile emission.
The following approaches can be made to control automobile emission:
1. Modification of internal combustion engines are needed to reduce the quantity of CO during fuel combustion.
2. Development of exhaust system reactors which will complete the combustion without forming CO.
3. Development of substitute fuels for gasoline which will give low concentration of CO on combustion.
4. Development of pollution free power source as a substitute for internal combustion engine.
5. Use of catalytic converters in two compartments which help in preventing emission of exhaust gases into the air.
2. Sulphur-dioxide (SO2):
Sulphur-dioxide is the second potent air pollutant as it accounts for 29% of the total weight of all pollutants.A. Sources:
There are two sources of SO2—
(1) Natural Source, and
(2) Anthropogenic source.
Natural processes like volcanic eruption provide 67% of the S02 pollution which is distributed all over the globe. Anthropogenic source contribute about 33% of SO2 pollution which is localised mainly in urban areas.
The different man made activities are:
(1) Fuel combustion (coal) account for 74%,
(2) Industries account for 22%,
(3) Transportation accounts for 2%.
Specifically, burning of fossil fuels in thermal power plants, manufacture of sulphuric acid and fertilizers, smelting industries etc, account for 75% of total SO2 emission while automobiles and refineries contribute to the rest 25%.
B. Sink of SO2:
The quantity of SO2 in atmosphere is very small as compared to its annual emission. This may be due to its reaction in atmosphere.
Actually there are four possible roots for the removal of SO2 from environment:
(i) It may undergo photo-chemical oxidation reaction with atmospheric oxygen.
(ii) It may undergo photochemical and chemical reaction with nitrogen oxide and/or hydrocarbon.
(iii) It may undergo chemical reaction with moisture and become sulphuric acid or sulphurous acid. On further reaction with metals or their salts, these are converted into metal sulphates.
(iv) It may undergo reaction with solid particles in the atmosphere.
C. Detrimental effects of SO2:
Sulphur dioxide is perhaps the most dangerous air pollutant affecting both living and non-living world.
(a) Effect of SO2 on man:
(1) It causes irritation to eye and respirating tract even at 2.5 ppm.
(2) It causes swelling of nasal system and stimulates mucus secretion.
(3) It causes lung cancer at high level.
(4) It induces desquamation or peeling off of the surface epithelium in the mucosa.
(5) Presence of S02 in moisture and fog becomes more dangerous due to formation of H2SO4 and H2SO3 which are 5 to 20 time more irritating than SO2 alone.
(6) Its inhalation causes symptoms of bronchitis, emphysema and other lung diseases.
(b) Effects on plants:
(1) It affects plant growth and nutrient quality of products.
(2) It kills leaf tissues causing leaf necrosis.
(3) It causes chlorosis and dwarfing when the concentration exceeds 1 ppm.
(4) Its chronic exposure to plants causes bleaching of plant pigments.
(5) At high concentration, it decreases PH of leaf tissues of some plants increasing the sulphur content ‘n plants.
(6) It affects stomatal pores, stomatal frequency, cholorplast and transpiration through stomata.
(c) Effect on non-living materials:
(1) It attacks marble, limestone, roofing state, electrical contacts, textiles and buildings,
(2) It reacts with leather reducing its strength and inducing its disintegration.
(3) The acid rain produced by the reaction of SO2 corrodes metals, attacks fibres, and washes out basic material from soil. The reaction of H2SO4 on marble is known as stone leprosy.
(4) It affects durability in paint films.
(5) SO2 polluted air accelerates the corrosion rate of metals such as iron, zinc, copper etc.
D. Control of SO2 pollution:
The oxides of sulphur can be reduced and controlled by the following methods:
1. Removing sulphur from fuel before burning.
2. Using low content sulphur fuels.
3. Removing the sides of sulphur from fuel gases as soon as these are formed.
4. Using non-sulphur containing fuels such as natural gases.
5. Using nuclear power to generate electricity from power plants.
3. Nitrogen Oxide (NOx):
NOx exists as nitrous oxide (N2O), nitric oxide (NO), nitrogen trioxide (N2O3), nitrogen peroxide (NO2) and nitrogen pentoxide (N2Os). Amongst all these nitrogen oxides, nitrogen peroxide (NO2) and nitric oxide (NO) are the two main reagents causing air pollution. About 95% of NOx is released to the atmosphere mainly as NO as remaining as NO2.a. Sources of Nox:
NOx is released to the atmosphere mainly through anthropogenic activities and partly through natural processes
1. Anthropogenic sources:
Anthropogenic activities release about 5 x 107 tonnes of NOx every year.
Some important activities include:
1. Combustion of coal, oil, natural gases and gasoline.
2. By-products of some chemical industries like HN03 am H2SO4.
3. Thermal power plants.
4. Supersonic aircraft exhaust.
5. Nuclear explosion.
6. Manufacture of nylon intermediates.
2. Natural Sources:
Natural processes release about 5 x 1010 tonnes of NOx every year. Some natural processes include:
1. Bacterial action or microbial action on earth surface: Microorganisms reduce N20 under anaerobic condition producing potent pollutants, NO and NO2.
2. Photo-chemical reaction in the atmosphere mediated by cosmic rays.
3. Volcanic eruption.
B. Consequences of NOx Accumulation:
The accumulation of NOx in the environment causes varieties of hazards such as acid rain, depletion of ozone layer, smog formation etc.
(a) Acid rain:
NOx causes acid rain by reacting with atmospheric moisture giving acids like nitric acid (HNO3) and nitrous acid (HNO2)
(b) Depletion of ozone layer:
NOx released to the atmosphere moves up and reacts with ozone layer causing its depletion. Such a process allows the passage of UV radiation into earth surface.
(c) Smog formation:
The presence of excess quantity of NO and NO2 induces smog formation and generates a number of harmful species like free radicals, PAN (Peroxy acetyl nitrate) etc.
C. Detrimental Effects of NOx on Living Organisms:
The accumulation of NOx is toxic to both plants and animals. The toxic action is attributed to their interference with the course of a number of biochemical reactions possibly through their modulating action over a number of cellular enzymes and production of a number of secondary pollutants like OH, HO2, O3, PAN etc.
(a) On plants:
Higher concentration of NOx causes:
1. Loss of photosynthetic activity.
2. Chlorosis
(b) On Human health:
The threshold limit value (TLV) of NO and NO2 for human beings are 25 and 5ppm respectively. Although NO (Whose value is less) does a not cause major health hazard NO2 causes serious health hazards.
Some serious health hazards are:
1. Inflammation of lung tissues.
2. Respiratory diseases like lung cancer, pulmonary haemorrhage.
3. Destruction of oxygen transport efficiency of blood.
D. Control of NOx Pollution:
In view of the detrimental and adverse effects of NOx on the entire living and non-living world, it is necessary to keep the level of NOx below the threshold value. Some important control measures are outlined as below:
1. To remove HOx from stack gases, one of the approach is the chemical absorption process (H2SO4 solution or alkaline shrubbing solution containing calcium hydroxide (Ca(OH)2) is used.
2. Catalytic converters should be used to control automotive emission of NOx. Catalytic converter acts in two ways:
(i) It decomposes HOx to nitrogen and oxygen in presence of suitable catalyst
(ii) It reduces NOx to nitrogen in presence of suitable catalyst and reducing agent.
3. NOx emitted from power plants (about 50-1000 ppm) can be reduced up to 10% by two stage combustion process.
(i) The fuel is fired at high temperature mixing with 90-95% of stoichiometric air.
(ii) Fuel burn out is completed at a relatively low temperature in excess of air.
4. Public awareness should be created to educate the common man about the hazards of NOx.
5. Government should take stringent action against industries discharging higher quantity of NOx into environment than the level prescribed by pollution control board.
Some more common chemical pollutants, then source and detrimental effects are described as in the table given below:
4. Hydrocarbons:
In addition to different gases, a series of hydrocarbons are released to the atmosphere either through natural processes or through human activities. These hydrocarbons sometimes behave like primary pollutants and some other times induce the formation of other potent secondary pollutants.a. Sources:
Natural sources of hydrocarbon release into air includes emission from trees, liberation from anaerobic decomposition of organic matter in presence of bacteria, liberation from domesticated animals etc. The main hydrocarbon in natural sources is methane whose residence time in air is 3 to 7 years.
Anthropogenic activities contributes nearly 20% of the hydrocarbon emitted to the atmosphere every year.
The major anthropogenic sources are:
(i) automobile exhaust,
(ii) industrial processes like processing, storage, transfer of products,
(iii) incinerator and refuse burning,
(iv) solvent , and
(v) burning of coal and wood etc.
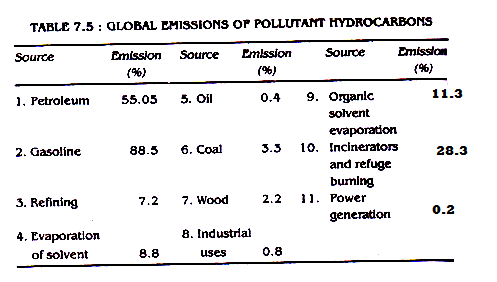
Global emissions of pollutant hydrocarbons is given in table 7.5.
Table 7.5 : Global Emission of Pollutant Hydrocarbons
b. Sinks:
The level of hydrocarbon in atmosphere is usually reduced by several reactions and photo-chemical reactions. Being thermodynamically unstable, these are oxidised in a series of steps to carbon dioxide. Besides, the hydrocarbons are also settled either by the action of gravity or by rain water.
c. Detrimental effects of hydrocarbons:
(a) Effect on human beings:
(i) It has carcinogenic effects on lungs due to its swelling at higher concentration.
(ii) Aromatic hydrocarbons causes irritation to muscus membrane.
(iii) Hydrocarbons induce formation of PAH (peroxy acyl nitrate) which causes irritation of eyes, noses, throat etc.
(iv) Excess of hydrocarbon causes blocking or respiratory tract.
(v) Benzpyrene induces cancer.
(vi) Methane at higher concentration causes narcotic effects.
(vii) Black-lung disease of coal miners, pulmonary fibrosis of asbestos workers and employsema of urban peoples are due to particulate accummulation.
(viii) The lodged particles in the lungs (<3µ) can cause severe breathing trouble by physical blockage and irritation of lung capillaries.
(b) Effects on plants:
(i) Hydrocarbons retard the growth of plants
(ii) These along with ozone cause chlorosis.
(iii) Ethylene hydrocarbons damage leaf tissues and flowering plants.
(iv) Acetylene and propylene bring about early maturity of plants.
(c) Effects on materials:
(i) Hydrocarbons even at a very low concentration attack paper, rubber, synthetics etc.
(ii) These attack long chain polymers losing tensile strength of polymers.
(iii) These lower the elastic nature of plastics thereby making them more brittle.
d. Control of Hydrocarbons:
The level of hydrocarbons can be reduced by controlling their emission from the source. The emission from automobile exhaust and industrial exhaust can be controlled through processes like incineration, absorption, adsorption, condensation etc.
5. Particulates:
Particulates are the small solid particles and liquid droplets present in the atmosphere in fairly large numbers. The dimension of particulates range from 0.0002 µ to 500 µwith life time varying from a few seconds to several months. The presence of these particulates in air causes serious environmental consequences.a. Sources:
The release of particulates into atmosphere is both natural and anthropogenic. The natural processes include volcanic eruption, blowing of dust and soil by wind, spraying of salt and other solid particles by the seas and oceans etc.
The contributions from manmade activities are: fly ash from power plants, melters and mining operations, smokes from incomplete combustion processes etc. The incomplete combustion processes are fuel combustion from stationary sources (wood, coal, oil, natural gas etc.), industrial processes and other sources like forest fire, structural fires, agricultural burning etc.
b. Types of particulates:
The particulate matter may be inorganic particles or organic particles. The inorganic particles include metal oxides (e.g. CaO, Fe3O4, V2Os, CaCO3 etc.), Metal halides (e.g. PbCl2, PbBr2, Pb BrCl etc.), salts (e.g. (NH4)2 SO4, CaSO4 etc.), fly ash asbestos particles etc. The organic particles are polycyclic aromatic hydrocarbons (PAH) such as benzo (α-) pyrene, Chrysene, benzofluoranthene, soot etc.
c. Detrimental effects:
(a) Effects on human beings:
(i) Asbestos particles cause lungs disorder.
(ii) Lead containing particulates affect children’s brain and interfere with the development and maturation of red blood cell.
(iii) Particulates of less than 1 µ reach alveoli of lungs and damage lungs tissues.
iv. Soluble aerosols get absorbed into blood from alveoli while the insoluble aerosols are carried to the lymphatic stream and get deposited in the pulmonary, lymphatic depot point or lymp glands.
(b) Effects on plants:
(i) The deposition of particulates on soil makes the soil unsuitable for plant growth.
(ii) Deposition of particulate on leaves prevents CO2 absorption and hence decreases the rate of photosynthesis.
(iii) Particulates deposited on plant leaves block the stomata of plants and thus inhibiting the rate of transpiration from the soil.
(iv) In case of some plants which are sensitive to traces of toxic metals, their enzyme activity is disturbed in presence of particulates containing trace elements.
(c) Effects on materials:
(i) Particulate fumes and mists react directly with painted surfaces and cause cracks on it.
(ii) Particulates induce corrosion of metals.
(iii) Particulates accumulate on the soil surface causing soil erosion.
(iv) Particles including fumes, dust, soot, mists and aerosols can bring about severe damage to buildings, sculpture and monuments.
(d) Effect of solar radiation:
(i) Particulates reduce visibility by absortion and scattering of solar radiation.
(ii) Particulates disturb the delicate heat balance of the atmosphere.
(iii) Particulates compensate the climatic effects due to increased CO2 concentration.
(iv) Particulates influence the climate through the formation of clouds, rains and snow, by acting as nuclei upon which water condensation can take place.
d. Control of particulate emission:
The quantity of particulates in the air can be minimised by the following techniques:
(i) By using gravity settling chamber.
(ii) By using cyclone collector.
(iii) By using cyclonic separators and trajectory separators.
(iv) By using filters and scrubbers.
(v) By using electrostatic precipitators.
Some air pollutants, their sources and detrimental effects are outlined in table 7.7
Table 7.7: Some Chemical Pollutants, Their Sources and Detrimental Effects
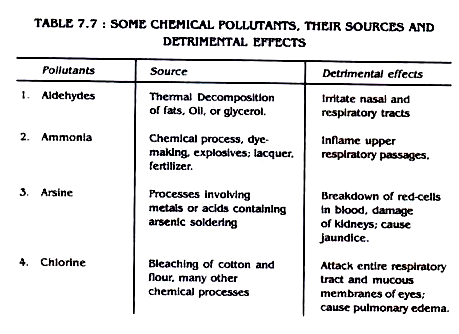
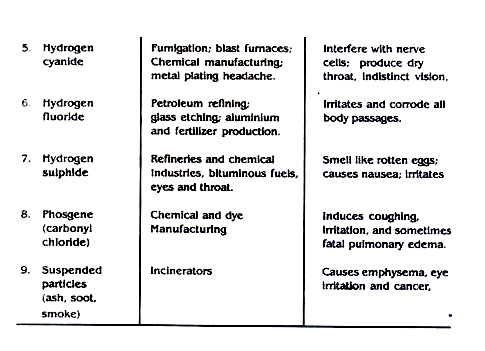
4. Effects of Air Pollution:
The important effects of air pollution are as follows: 1. Atmospheric particles, due to fuel combustion for industrial and household heating purposes, can scatter and absorb sunlight and thus reduce visibility.
2. Increased level of CO2 causes greenhouse effect.
3. Chlorofluoro carbons (CFCs) and nitrogen oxides cause ozone layer depletion and ozone hole.
4. The effect of particulate matter includes corrosion of metals, erosion and soiling of buildings, sculptures and painted surfaces and soiling of clothing and draperies, damages of electric equipment’s etc.
5. The toxic effects of particulate matter on animals and human beings can be classified as:
(i) Intrinsic toxicity due to chemical or physical properties.
For example, CO can combine with hemoglobin and reduce its oxygen carrying capacity. Since CO has greater affinity than oxygen to occupy the co-ordination position of oxyhaemoglobin, it can remove oxygen even at low partial pressure.
HbO2+ CO→ HbCO + O2
Interference with clearance mechanism in the respiratory tracts. For example, chronic bronchitis and emphysema have also been found to be caused by SO2. Toxicity due to absorbed toxic substances. For example, lead particles from vehicle exhaust, in higher dose, can kill outright but in lower dose shortens life span and causes deterioration of nervous system.
6. The oxides of sulphur and nitrogen combine with water vapours of the atmosphere and cause acid rain.
7. Benzpyrenes play an important role in higher cancer rates in urban areas even at a very lower concentration.
8. The small solid particles can serve as carrier for microorganisms and other infective agents and thereby spreading diseases.
5. Consequences of Air Pollution:
The accumulation of pollutants in air causes a number of disasters to the living world. Some important consequences of air pollution are as follows: A. Ozone Layer Depletion:
Ozone layer present in the stratosphere strongly absorbs UV radiation of 220-203 nm and thereby protects life on earth from severe radiation damage. But due to continuous and non- interrupted release of chloroflurocarbons, oxides of nitrogen etc. by the civilised world, the ozone layer is constantly depleted. The reaction is medicated through a number of free radicals like CI+, CIO+, + OH etc. and a number of portent species like (O, NO, SO2 NO2 etc.) The mechanisms of some important reactions causing ozone depletion are given below.(i) Depletion through CFCs:
CFCI3 hv (200nm) → CFCI2+ CI+
CI+ + O3→ CIO+ + O2
CIO+ + O→ Cl+ + O2
Each CFC is capable of destroying 1 lakh of 03 molecules in chain reactions. It is also calculated that 1 kg of CFC can deplete 3.5 tonnes of ozone.
(ii) Depletion through MO (Nitric oxide)
NO + O3→NO2 + O2
Since residence time of NO in atmosphere is longer, it is a potent agent causing depletion.
(iii) Depletion through N02 (nitrogen peroxide)
2NO2 + O2→ N2O3 + 2O2
(iv) Depletion through Cl2 gas:
Cl2 hv (300- 400nm) →2Cl+
CI+ + O3→ ClO++ O2
CIO++ O→ CI+ + O2
(v) Depletion through nascent oxygen:
O + O3→ 202
(vi) Depletion through +OH (Hydroxyl radical)
+OH +O3→O2 + HOO+
(vii) Depletion through SO2
SO2+ O3→SO3 + O2
(viii) Depletion through N2 O (Nitrous oxide)
N2O + O3→ 2NO2
Due to the depletion of ozone layer, the harmful solar radiations easily penetrate into the environment and cause the following detrimental effects:
Ozone Hole:
In the early sixties (1963) scientists have reported a large hole in the ozone layer over Antarctica where ozone level dropped by 30 per cent. The ozone hole covers an area as large as USA. CFCs were considered to be prime suspect for causing ozone depletion.
It was also established that one molecule of CFC is capable of destroying 3 lakh ozone molecules. Subsequently a similar hole was discovered over the thickly populated northern hemisphere. A study by NASA scientists (National Aeronautics and Space Administration, USA) revealed that the area of ozone over northern hemisphere decreased by 3 per cent between 1969 and 1986.
The overall reduction in the O3 layer is now estimated to be about 8 per cent. Under the auspicies of the United Nations Environment Programme (UNEP), 34 countries have signed an agreement at Montreal, Canada, in 1986 called “Montreal Protocol”. The scientists have agreed to reduce the production and use of CFCs upto 50 per cent by the year 1999. In a second meeting at Helsinki held in 1989, it was agreed upon a total phaseout of CFCs by 2000 A.D.
Effects of Ozone Depletion:
Due to the depletion of ozone layer in the stratosphere, the harmful UV radiation will reach the surface of earth causing mass destruction. Some of the harmful effects of UV-radiation may be outlined as below:
1. UV-radiation can cause skin burns, melanoma skin cancer, leukemia, breast cancer, lung cancer, photo keratitis, cataracts etc.
2. UV-radiation may damage the genetic material, DMA leading to mutation.
3. UV-radiation injures plant proteins and causes depletion of chlorophylls and mutations.
4. The depletion of ozone layer will induce eye cancer in cattle.
5. The depletion of ozone layer leads to the loss of various plants from terrestrial and aquatic habitats.
6. It will reduce the crop productivity.
7. It will bring about significant changes in the climate.
8. Due to depletion of ozone layer, UV-radiation may cause Green House effect changing the global energy.
Protection of ozone layer:
By protecting the ozone layer of the stratosphere, we can protect the human beings, all plants and animals, eco-system and finally biosphere from destruction. Some protective and controlling measures of ozone layer depletion may be outlined as given below:
1. The production and use of CFCs should be banned and their substitutes should be found out.
2. The use of plastic foam has to be boycotted.
3. The use of CFCs in aerosol, spray cans, egg crates etc. should be banned and available substitutes be used.
4. Suitable procedures should be adopted to recapture the CFCs released from the air-conditioner and refrigerator servicing units.
5. Stringent international and national laws are to be promulgated to see that ozone layer remains intact without destruction.
Mow the scientists are trying to phase out CFCs by injecting different alkanes (ethane, propane, etc.) into Antarctica Atmosphere. These alkanes are capable of capturing the chlorine radicals, responsible for ozone depletion obtained from CFCs.
(i) It causes skin cancer, breast cancer, lung cancer, eye cataracts etc.
(ii) It causes damage of the genetic materials like DHA and RNA leading to mutations.
(iii) It inhibits the protein synthesis and causes the depletion of chlorophyll in plants.
(iv) It induces eye cancer in cattle.
(v) It reduces the crop productivity.
B. Green House Effect:
The green house is that body which allows short wavelength solar radiation to pass through it but does not allow the long wavelength infrared radiation to escape. Due to rapid and unplanned industrialization the gases like CO2, chlorofluorocarbons (CFCs), methane (CH4), nitrogen oxides (NOx), ozone (O3)/etc. accumulate in the atmosphere. The layer of these gases behave like wall of a green house and transmit short wave solar radiations but does not allow the longer wavelength heat radiation (infrared) to be reflected back into outer space. That is green house are transparent to solar radiation but not to heat radiation.Thus, the green house effect may be defined as the progressive warming up of the atmosphere at the surface of earth due to blanketing of infrared radiation from the earth’s surface by the green house gases.
(G.H. gases) + hv (Solar radiation) → (G.H. gases) + hv (solar radiation) (G.H. gases) + hv1 (infrared) → (G.H.gases)+
The green house effect is based on principle of infrared absorption characteristic of gases. AH the green house gases are infrared sensitive. It is seen that higher the concentration of green house gases, higher will be the magnitude of IR-radiation trapped and re-emitted back to earth’s surface increasing the mean global temperature. The contribution of different gases inducing green house effect is as follows:
(i) CO2→50%; (ii) CFCs→18 %;( iii) CH4→14%; (iv) NOx→5 to 7%; (v) O3→3 to 5%.
The intensity of the effect increases in presence of dust, aerosols, etc.
The green house effect was for the first time suggested by J. Fourier (1827). This effect is also known as Global Warming or Carbon dioxide enrichment or atmospheric effect. The green house effect was initially essential for colder climates to grow few plants in winter, which require higher temperature for their growth and survival. But, now-a-days, it is seen that enrichment of green house inducing gases in the atmosphere also cause global warming because their concentration is far above the normal. The fundamental principles underlying green house effect are:
(i) Absorption of infrared radiation by the green house inducing gases,
(ii) Re-emission back toward the earth surface which results in heat trap and increases the mean global temperature.
Sources of green house gases:
Some major sources of green house inducing gases may be described as given below:
(i) CO2 gas is released into the atmosphere by the burning of fossil fuels (coal, oil, natural gases etc.), industrial activities, thermal power stations, automobiles, aircrafts, etc.
(ii) CFCs and halons are released during the operation of coolants and refrigerant, burning of plastic foam products and from spray cans.
(iii) Methane (CH4) gas is released from domestic waste and sewage.
(iv) Reduction of ground cover and deforestation indirectly enrich CO2 gas in the atmosphere.
(v) Nitrous oxide (N2O) is released to the atmosphere due to forest Fire, burning of grassland and natural oil and bio-decomposition of nitrogenous fertilisers.
(vi) The concentration of ozone in the atmosphere increases due to the formation of photo-chemical smog.
Effect of Green House Gases on Climate Change:
A number of predictions have been made about the change in global climate due to green house effect. Some of these may be outlined as given below:
1. It is reported that between 1960 and 1986, the average global level of CO2 has increased by 26 per cent (from 275 ppm to 346 ppm. and this level may reach upto 550 ppm by the end of next century). Such an increase in CO2 concentration will lead to an average increase in global temperature by 1.5°C to 4.5°C. Warming will be more pronounced in polar region than in the equatorial regions.
2. Increase in temperature in the poles will result in the melting of glaciers and polar ice caps and raising of the level of sea water by about 1.5 metres.
3. Increase in temperature will evaporate terrestrial water content leading to shortage of drinking water.
4. Increase in temperature changes the rainfall pattern.
5. The snowing period will be reduced as a result of which the rate of decomposition of organic matter will increase.
Effect on Agriculture:
Global warming has significant impact on agricultural productivity. Some of these effects are discussed as given below
1. Global warming will decrease in cereal production of the world due to reduction of soil moisture. However, the productivity may increase in the polar region.
2. The changes in climatic conditions may shift the cropping pattern.
3. An increase in temperature will increase the rate of development of insect pests.
Effect on Human Health:
Global warming has significant effect on human health and human diseases:
1. It may favour spreading of diseases like malaria and filaria.
2. It may favour the breeding and growth of insect vectors.
3. It may favour the spreading of insect vectors from one latitude and altitude to other, thereby extending the boundaries of the diseases.
Control of Green House Effect:
In order to reduce the concentration of different green house gases including gases in the atmosphere and to cope with green house effect, the following strategies should be adopted :
1. Reduction in the consumption of fossil fuels drastically,
2. Increase of the efficiency of internal combustion engines used in automobiles so that the pollutants in the exhaust will be reduced.
3. Use of scrubbers to remove CO2 from the emissions of coal burning power plants and industries.
4. Methanol as a substitute to be used in transport sector.
5. Use of biogas for domestic purposes, as conventional energy.
6. Use of unleaded petrol in vehicle.
7. Banning of the use of Chlorofluorocarbon (CFCs).
8. Banning of deforestation.
9. Developing greeneries by undertaking massive afforestation programmes.
Smog:
Smog is a combination of two words smoke and fog. The word smog was for the first time suggested by H. A. Vocus in 1905 but firstcame into limelight in Belgium in 1930 as smoky fog. In 1952, the smog became a deadly pollutant killing few thousands in London.Smoke is produced as a result of the incomplete combustion of fuels. The smoke contains various gases and suspended particulate matter. In addition, another substance responsible for smog formation is dust. Dust is nothing but small particle of solid matter that can be carried in suspension. Dusts are produced from forest fires, automobile exhaust, industrial combustion process, soil blowing by wind, ocean spray, mining areas, etc.
Dust combines with smoke and later with fog to produce ‘smog’, which appears in the form of cloudy layer in the atmosphere. A thick cloud of smog is produced where smoke instead of escaping, the area to outer atmosphere stays nearer to the surface due to temperature inversion.
The smog is caused either due to the presence of oxidising pollutants or due to reducing pollutants. If the smog is caused due to oxidising pollutants, it is known as oxidising smog and if it is caused due to reducing pollutants, it is known as reducing smog. Besides, the smog can also be classified according to its pollutant content or nature of the reactions shown by the pollutants or the source from which the pollutants are generated etc. Let us discuss various types of smogs and their characteristics:
1. Sulphurous Smog:
It is a mixture of fog, smoke and sulphur dioxide (SO2) gas. This type of smog was marked in London in 1952 where the smog prevailed for five days killing few thousands. The pollution was caused due to sulphur dioxide (SO2) and smoke during temperature inversion. The effects were more severe in morning hours and became deadly after sunrise because of photochemical oxidation. The smog caused bronchitis, pneumonia, respiratory distress, and eye diseases. Since it is a mixture of reducing pollutants, it is also known as reducing smog.
S+O2 hv→ SO2
SO2+ O → SO3
SO2 + H2O→H2SO3
H2SO2+O→H2SO4
H2SO4+2NH3→ (NH4)2 SO4
2. Los Angeles Smog:
Such type of smog was recorded at Los Angeles in 1944 and thereafter it’s known as Los Angeles smog. It is a type of photo-chemical smog. The smog contains different compounds at different times of a day. In the morning, when nitric oxide (MO) is discharged from automobile exhausts build up and react with oxygen to form nitrogen dioxide (NO2) which is a yellowish brown gas with pungent and chocking odour. Since MO2 gas produces a characteristic brown haze, the cities where this type of smog predominates, are called brown air cities.
As the sun rises, the UV-rays cause a rapid conversion of NO2 to MO and nascent oxygen atom. The peak is obtained in- between 10 A.M., and 4.00 P.M. The nascent oxygen atoms react with oxygen molecules of air to give Ozone (O3), the concentration of which is maximum at around 10 A.M. The other highly reactive chemical forms are hydrogen peroxide (H2O2), hydroxy radicals (OH) etc.
The presence of oxides of nitrogen in the environment induces the formation of other potent species like ozone (O3), hydrogen
Peroxide (H2O2), hydroxyl radical (HO+), carbony radical (R CO+), acetly peroxy radical (CH3 COOO) + etc.
The smog brings about a disaster in the living world possibly through the species like ozone, H2O2, hydroxyl radicals (+OH), acetyl peroxy radicals, peroxy acyl nitrate (PAN), hydroperoxy radical etc. The accumulation of oxides of nitrogen in the environment induces the formation of other potent species as per the given mechanism.
NO2 hv (traction)→ NO + O
NO + O2 Uv-ray→ NO2 + O
H2O+ O → H2 O2 (Hydrogen peroxides)
H2O2 →2+OH (Hydroxy I radical)
H2O2→ H+-O-O (Hydroperoxy radical)
O2+ O → O (Ozone)
HO+CO +O2→Co2H-O-O+
R- CO- H + + OH → R-CO+ + H2O
R- CO+ + O2→R-CO – O- O+ (Acyl peroxy radical)
R- CO – O –O ++ NO2→ R- COO – NO2 (Peroxy acyI nitrate)
R- CH2-H + +OH →R- CH2+ + H2O
R – CH2+ + O2 → R- CH2– O- O +
R- CH2– O – O+NO2→ R- CH2 –O+ + NO2
R – CH2-O+ O2→ R – CHO + HO – O +
H – O- O+ + NO → NO2+ + OH
R- CH2– H +OH →R – CH2+ H2O
Effects of Smog:
The formation of smog is highly destructive and influences both the physiological and metabolic activities of living organisms’ some important effects of smog are discussed as given below:1. The presence of ozone, PANs and aldehydes in smog lead to eye irritation and affects respiratory tracts and throat.
2. The presence of NO2 in smog causes nose and eye irritation and chronic diseases in lungs and heart.
3. The presence of PANs in smog causes extensive agricultural and forestry damage, such as damage to leave and stomatal tissues.
4. PANs cause dizziness and headache in man.
5. Smog includes early maturity of plants and hence induces senescence and reduces rate of photosynthesis.
6. Smog damages metals, paper, rubber and fabrics.
7. The particulate matters in smog induce lung cancer.
8. Ozone decolourises the paintings.
Control of smog:
The following are the methods to control smog:1. The production of nitrogen oxide (NOx) and hydro-carbon should be controlled which checks the production of ozone and PANs.
2. The process like incineration, absorption, adsorption and condensation should be employed to reduce the different harmful constituents of smog such as oxide of sulphur and nitrogen, hydrocarbon, carbon-monoxide, dust etc.
Acid Rain:
Now-a-days, one of the major consequences of environmental pollution is the acid rain. It has become serious threat to water bodies like ponds, rivers, lakes and reservoirs and also to the terrestrial eco-systems like grasslands and forests. Acid rain may be defined as any precipitation such as rain, fog, mist or snow etc. which is more acidic than the normal i.e. having a lower pH than that of normal rain water. Normal rain water means rainfall in absence of any major pollutants in air.The normal rain water is also slightly acidic (pH approximately 5.6) because the basal level of CO2 gas present in the air can also be solubilised in rain water giving carbonic acid (H2CO3), weak acid. However, when the atmospheric air is highly polluted containing the major pollutants like oxides of sulphur and nitrogen and halogen radicals, these pollutants react with water vapours or rain droplets to form different types of strong acids like sulphuric acid (H2SO4), nitric acid (HNO3) and hydrochloric acid (HC1) which have grave environmental impact. Hence, acid rain means the presence of excessive strong acids in rain water which lowers the pH of water from normal.
The term acid rain was first referred by Robert Angus in 1872. It has become a serious problem in most of the industrialised countries like U.S.A., U.K., Germany, Norway, Sweden, Russia, Canada, etc. In India, it is also known that acid rain is destroying the greatest monument Taj Mahal in Agra. Here, the acid rain is caused due to Mathura Oil Refinery, an industry discharging a number of poisonous gases into the atmosphere.
Source of acid rain:
Acid rain is due to the presence of various pollutants like oxides of nitrogen, oxides of sulphur and halogen radicals or molecules in the atmosphere. These pollutants are discharged into the atmosphere by several natural processes and human activities.1. Natural Sources:
Natural phenomena like volcanic eruptions, forest fires, lightening, burning of fossil fuel, decomposition of organic matters etc release large quantities of these pollutants into the atmosphere.
2. Human Activities:
Human activities like burning of fuels, automobile exhausts, industries and smelting plants, thermal power plants, petroleum refineries etc. release large quantities of these pollutants into the atmosphere.
Formation of acid rain:
After the release of the pollutants (oxides of sulphur and nitrogen and halogens) into the atmosphere, these can travel thousands of kilometres. The longer these stay in the atmosphere, the more likely these are to be oxidized into acids. The conversion of the pollutants into their corresponding acids are shown in theEquations given below:
(a) Formation of Nitrogenous acids:
N2 + O2→ 2NO Nitric oxide
NO + O3→ NO2 + O2 Nitrogen peroxide
2NO2 + O → N2O3+ 2O2 Nitrogen trioxides
2NO2 + O → N2O5 Nitrogen pentoxide
N2O5 + H2O → 2HNO3 Nitric acid
N2O3 + H2O→ 2HNO2 Nitrous acid
(b) Formation of Sulphuric acid:
S + O2 → SO2 Sulphur dioxide
2SO2 + O2→ 2SO3 Sulphur trioxide
SO3+ H2O→H2SO4 Sulphuric acid
SO2 + H2O→ H2SO3 Sulphurous acid
(c) Formation of Hydrochloric acid:
Cl2 hv→ Cl + + Cl +
H2 hv→ H+ +H+
H+ Cl+→ HCl
(d) Formation of carbon acid:
CO2 + H2O →H2CO3 Carbonic acid
All these acids with naturally occurring carbonic acid (H2CO3) form acid rain with rain water and pH falls to 4,0, at times the pH can also fall to 2.0. The concentration of these acids as well as the quantity of water in which the acids are dissolved determines the pH of rain water. Heavy rains are usually less acidic as there is relatively more water. On the other hand, fogs and mists are more acidic as the acid molecules are dissolved in relatively little water.
Effects of Acid Rain:
Acid rains create complex problems and their imports are far reaching. Some major impacts of acid rain on the eco-system are discussed below:1. Acid rain increases the soil acidity affecting land flora and fauna.
2. It causes acidification of aquatic bodies which leads to the killing of aquatic plants and animals. The increase in acidity also affects the metabolism, growth and development of aquatic organisms.
3. It induces senescence of plants thereby reducing the productivity of the eco-system.
4. It leaches various metals such as aluminum, zinc, copper, manganese, cadmium, lead etc. from the soil into aquatic bodies. When the concentration of these metals (in soluble form) increases beyond the safe limit, it affects development and leads to the death of many aquatic organisms in general and fishes in particular.
5. It corrodes buildings, monuments, statues, bridges, fences, railings etc.
6. Acid rain affected plants and animals are easily attacked by pathogens.
7. Diseases caused by bacteria and pathogens can be spread by acid rain water.
8. Acid rain may cause respiratory and skin diseases.
Some important air pollution disasters are given in Table 7.8.
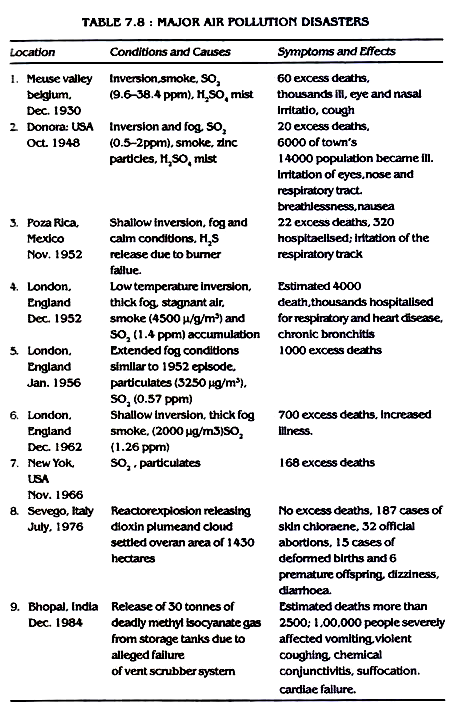
Control measures:
Acid rain can only be controlled by managing the source of pollution. Several control methods are adopted to manage acid rain.Some important control measures are as discussed below:
1. The aquatic bodies and farmlands should be periodically limed to neutralise the acidity due to acid rain.
2. The fuels, devoid of sulphur or having low sulphur amount should be used to minimise the quantity of sulphur dioxide gas in the atmosphere.
3. The leakage of chlorine or its discharge should be stopped.
4. The vehicular exhaust should be minimised by using control valves in the outlet of the exhaust pipeline of the automobiles.
5. The scrubbers should be used to reduce the emission of SO2 during coal burning.
6. SO2 gas released is sprayed with water containing lime which precipitates SO2 as calcium sulphate (CaSO4).
7. General public awareness should be created regarding the ill effects of environmental pollution and consequences of acid rain.
6. Control of Air Pollution:
In view of the above detrimental impacts of air pollution on mankind, plants and vegetation, we have to adopt some control measures in order to reduce the level of air pollutants in environment. Some important control measures are as follows:
(i) Pollution from domestic fire should be controlled to the house holder’s use. Alternative methods of the fuels like smokeless chullah should be used in order to increase the fuel efficiency and reduce the emission of solid particulates.
(ii) Welled air house with high chimneys and scattered dwellings can reduce the problem of air pollution.
(iii) As the plants absorb mainly air polluting gases like CO2, there should be massive afforestation programme in industrial areas and big cities.
(iv) Setting of air quality standard for protection of environment and human health is highly essential. Ventilation and air-conditioning should be introduced.
(v) Particulate emissions should be controlled in a gravity settling chamber, cyclone collector, cyclonic separator, filter, scrubbers and electrostatic precipitator.
(vi) More and more flyovers, bridges, bylanes, footpaths for pedestrians, steamers and ferry launches and other modes of navigational metropolitans public transport systems should be developed with a view to minimize the usage of individual auto vehicles and thereby smoke emissions. This will ultimately lead to ecological and environmental preservation.
(vii) More and more diesel multiple unit trains (DMU Trains) should be introduced in town area so that the people’s dependency on the individual and public road transport may be minimized.
(viii) Environmental education and awareness programmes need to be institutionalized by incorporating these in the essential activities of educational institutions.
(ix) Use of unleaded petrol and diesel should be made compulsory.
(x) Adequate legislation (Air Act) should be enacted to compel to control air pollution. Severe punishment should be specified to the defaulters.
(xi) The use and production of CFCs should be banned with suitable substitute in order to check air pollution.
(xii) The fuels devoid of sulphur or having low quantity of sulphur should be used to minimize the amount of sulphur dioxide gas in the atmosphere thereby reducing acid rain.
(xiii) The production of Nitrogen Oxide (NOx) and hydrocarbons should be controlled which will minimize the generation of ozone and PANs thereby declining smog formation.
(xiv) The vehicular exhaust should be minimised by using control valves in the outlets of the exhaust pipeline of the automobile.
Air Quality Standards:
Air quality shows a combination of the physical and chemical characteristics which makes air, an important resource for better existence of all living organisms including man. The physical characteristics are described by factors like temperature, density, moisture content and air movement in the troposphere. The chemical characteristics include the concentration of different gases and pollutants.Mainly the natural processes are responsible in altering the physical characteristics whereas changes in chemical characteristics are due to anthropogenic activities. The emissions from automobiles, industries, agricultural processes etc. add a number of obnoxious gases which adversely affect the air quality and render it unfit for living organisms on earth.
It is, therefore, necessary to know to what extent the pollutant present in air can result in a situation in which the latter can be termed as polluted. The Pollution (Prevention and Control) Boards, of different countries have fixed standards for ambient air quality (in India under Air Act 1981) beyond which an ambient air can be considered as polluted. In India, the Air Act 1981 prescribes emission standards for many industries.
Thus, air quality standards is a limit on the amounts of a given pollutant permitted in the air around us and emission standard signifies the maximum amount of pollutants those can discharged from a specific point source. Till recently, limited, attention has been focused on vehicular pollution control in India.
The environment (Protection) Act, 1986 puts the responsibility of laying standards for vehicular emission to the Central and State pollution Control Boards so that standards can be incorporated in motor vehicle Acts and Rules.
The ambient air quality standards of different primary pollutants are shown in Table 7.9.
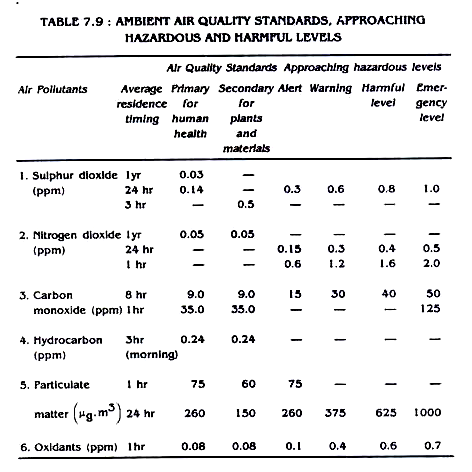
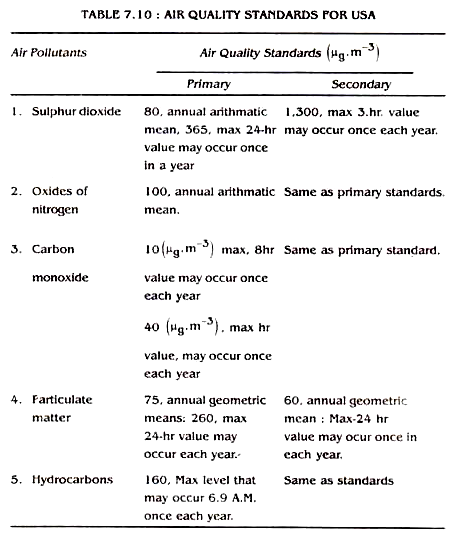
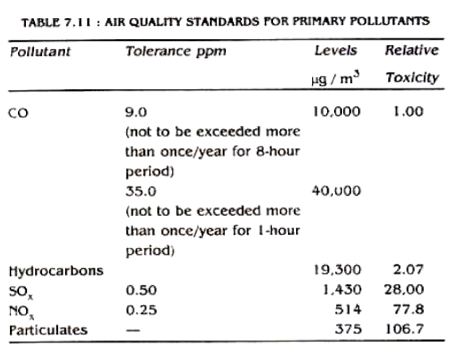
The governments of many countries have their legal standards for ambient air quality. The purpose of these standards is to reduce the pollutants to a certain level which would avoid undesirable effects. The prescribed standards may vary slightly from country to country depending upon their meteorological and geographical features and the population density. The air quality standards for United States of America are given in Table 7.10. and 7.11.