Why is the ocean blue?
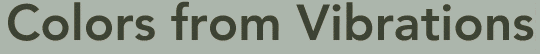

Crater lake, Oregon, USA, is widely known for its intense blue color and spectacular view. The appearance of the lake varies from turquoise to deep navy blue, depending on whether the sky is hazy or clear.

The turquoise color of Garibaldi Lake, British Columbia, Canada.
Why is water blue?
Water’s intrinsically blue color is easy to see when the water is sufficiently deep, such as in the Caribbean and Mediterranean Seas, and in Colorado mountain lakes. Pure water and ice have a pale blue color, which is most noticeable at tropical white-sand beaches or in ice caves in glaciers. (Green colors are usually derived from algae.) The blueness of the water is neither due to light scattering (which gives the sky its blue color) nor dissolved impurities (such as copper). Because the absorption that gives water its color is in the red end of the visible spectrum, one sees blue, the complementary color of orange, when observing light that has passed through several meters of water. Snow and ice has the same intense blue color, scattered back from deep holes in fresh snow.Blue water is the only known example of a natural color caused by vibrational transitions. In most other cases, color is caused by the interaction of photons of light with electrons. Some of these mechanisms are resonant interactions, such as absorption, emission, and selective reflection. Others are non-resonant, including Rayleigh scattering, interference, diffraction, and refraction. Unlike with water, these mechanisms rely primarily on the interaction of photons with electrons.
The bent water molecule H2O in the free state has three fundamental vibrations. It is helpful to think of metal spheres fixed on strong springs in visualizing these vibrations. The three normal modes are: (a) the symmetrical stretch, (b) the symmetrical bend, and (c) the antisymmetrical bend.

The faint blue color of water is seen in this photo. Here, you look upwards through 3-meter long sealed aluminum tubes filled with purified water. On the left, the faintly bluish tube contains regular (light) water, and at right, the clear tube is empty.
Why vibrational?
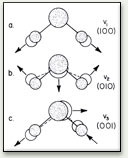
Water owes its blueness to selective absorption in the red portion of its visible spectrum. The absorbed photons promote transitions to high overtone and combination states of the nuclear motions of the molecule, i.e. to highly excited vibrations. We know molecular vibrations color water because "heavy" water (which is chemically the same as regular water, but with the two hydrogen atoms replaced with deuterium atoms - an isotope of hydrogen with one extra neutron that makes "heavy" water about 10% heavier) has a similar absorption curve, shifted to higher wavelengths outside of the visible spectrum of light. Heavy water is thus colorless.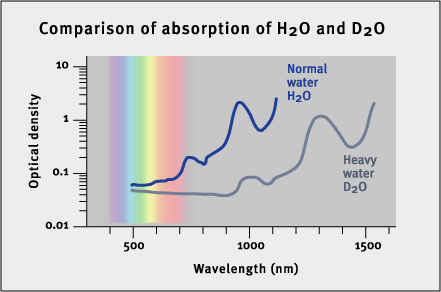
These graphs illustrate why water (H2O) is blue, while "heavy" water (D2O) is colorless. The graph gives the visible and near-IR spectrum of H2O and D2O at room temperature. The absorption below 700 nm in wavelength contributes to the color of water (the blue graph). This absorption consists of the short wavelength tail of a band centered at 760 nm, and two weaker bands at 660 nm and 605 nm. The vibrational origin of this visible absorption of H2O is demonstrated by comparison with the spectrum of heavy water, D2O (the gray graph). Heavy water is chemically the same as regular (light) water, but with the two hydrogen atoms (as in H2O) replaced with deuterium atoms (deuterium is an isotope of hydrogen with one extra neutron - the extra neutron that makes "heavy" water about 10% heavier). Heavy water is colorless because all of its corresponding vibrational transitions are shifted to lower energy (higher wavelength) by the increase in isotope mass. For example, the H2O band at 760 nm (the red end of the spectrum) is shifted to approximately 1000 nm in D2O. This is outside the spectrum of visible light, so heavy water has no color.
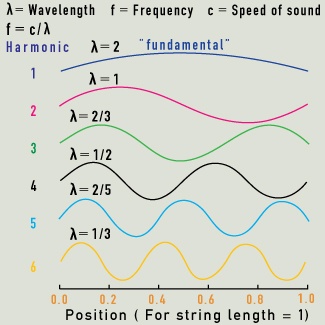
Overtones (also called harmonics) are secondary vibrations of the string, with wavelengths in integer ratios to the fundamental note.
What is the role of overtones?
In music, a note has a fundamental wavelength and pitch that depend on the nature of the vibrating air column or string. A violin string’s pitch depends first on its vibrating length, and then on its thickness and tension. Secondary notes linked to this fundamental pitch are created when the string vibrates as though split into halves, thirds, quarters, and so on. The overtones have higher pitches than the fundamental pitch, and the note we hear is a combined sound, enriched by the overtones. The relative strength of the fundamental and overtone pitches contributes to the unique sound we associate with each instrument.Molecular vibrations also have overtones related to their fundamental wavelength. Just as we hear a musical note that is a combination of a fundamental note with its overtones, so molecules may vibrate in complex combinations of their fundamental and overtone vibrations. In water molecules, only the first few overtones make a significant contribution to the overall vibrational energy.
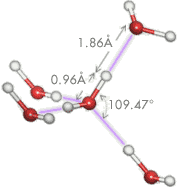
Hydrogen bonding (purple) is a special type of dipole-dipole bond that exists between an electronegative atom and a hydrogen atom bonded to another electronegative atom. In water, the hydrogen atom (white) is covalently attached to the oxygen (red) of a water molecule (about 470 kJ/mol) but has an additional attraction (about 22 kJ/mol) to a neighboring oxygen atom of another water molecule. Hydrogen bonding is weak compared to covalent and ionic bonding.
What is the role of "hydrogen bonding"?
Water is unique among the molecules of nature in its high concentration of O-H bonds and in its plentiful supply. Most importantly, the O-H symmetric (v1) and antisymmetric (v3) vibrational stretching fundamentals are at high enough energy so that a four-quantum overtone transition (v1+ 3v3) occurs just at the red edge of the visible spectrum. When comparing the vibrational transitions of gaseous and liquid water, the liquid phase O-H stretching band is red-shifted (to a lower energy) from the gas phase values of v1 and v3 by several hundred wavenumbers. This shift is primarily the result of hydrogen bonding in the liquid. The near-IR absorption bands of ice (solid phase) are the most red-shifted of all. Hydrogen bonding in water causes the stretching frequencies of H2O to shift to lower values. It is believed that if water did not have hydrogen bonds, it would still be colored, perhaps with a more intense blue than actual water.
Other hydrogen-containing liquids and solids besides water, such as liquid ammonia, could possess traces of bluish color because of vibration and rotation effects. However, water and ice are the only two chemical substances occurring in sufficiently large bulk for a weak coloration to be visible.
Why do we not see colors caused by molecular vibrations in many other substances?
Most molecules have vibrational energies that are lower in frequency (longer in wavelength) than that of water, falling in the range of far infrared or thermal vibrations rather than in the visible light range. The hydrogen atoms in water are very light, and the bonds between hydrogen and oxygen very strong, which shifts them to higher frequencies (with shorter wavelengths), with overtones that lie in the range of visible light. Just as the pitch of a vibrating string is raised if the mass of the string is reduced and the tension applied to the string is increased, so too the highest-frequency vibrations occur with the lightest atoms (hydrogen) when most strongly bonded (to oxygen in water).Vibrational states, as well as the related rotational states, also can modify the energy of the electronic excitations and are involved in the violet color of iodine vapor, the reddish-brown of bromine, and the green of chlorine.
 Purple iodine vapor is produced by heating iodine crystals (combined electronic-vibration-rotation color). |  The blue green light of natural gas burning on a kitchen burner emitted by an oxygen-rich gas flame as seen on a kitchen range also involves such combination vibrational, rotational, and electronic excitations in the unstable molecules CH and C2. |