The Constitution of India came into force on 26th January, 1950. Originally, the constitution contains no specific provisions for environmental protection. However, certain specific provision have been incorporated by the Constitution (Forty Second Amendment) Act, 1976 and subsequent amendments. Indian Constitution is one of the very few constitutions in t he world, which provides for specific provision for the protection and improvement of the Environment.
The constitution, being the fundamental law of the land has a binding force on citizens, non – citizens as well as the State. The Fundamental Rights and the Directive Principles of the State Policy underline our national commitment to protect and improve the environment. The courts in India have also given a new interpretation to the constitutional provision relating to protection and improvement of the environment (the intended meaning of the environment in the constitution) may be explained with reference to the following head:
1. The Constitution Forty Second Amendment.
2. Federal System of Govt. (Distribution of Legislative Power).
3. Fundamental Rights.
4. Directive Principles of State Policy; and
5. Fundamental
1. Constitution Forty Second Amendment: - In 1976, under the leadership of the then Prime Minister, Smt. Indira Gandhi, the Constitution (Forty Second Amendment) Act was passed and the provisions relating to the protection of environment for the first time were incorporated by adding a new provision Article 48-A in the Chapter, Directive Principles of State Policy.
According to Article 48-A “the State shall Endeavour to protect and improve the environment and to safeguard the forests and wildlife of the country”.
Further, a new provision Article 51-A in the form of “Fundamental Duties” was also incorporated by the 42nd Amendment. According to the sub-clause (g) of Art. 51-A, “it shall be the duty of every citizen of India to protect and improve the natural environment including forests, lakes, rivers and wildlife and to have compassion for living creatures”. The above two provision impose two-fold responsibilities. On the one hand, it gives directive to the State for protection and improvement of environment, and on the other hand it casts/imposes a duty on every citizen to help in the preservation of natural environment.
2. Federal System of Government (Distribution of Legislative Power):-
From environmental point of view, allocation of legislative authority is very important. The constitution of India deals exhaustively with legislative powers pertaining to environmental law. The legislative powers under the scheme of the constitution is divided into three lists viz., the Union List or List – I, the State List or List –II, the Concurrent List or List – III. Part – XI (Arts. 245-263) of the constitution provides for the distribution of legislative powers between the union and the states. Article 246 distributes the subjects of legislative power in these three lists between the Centre and the States. The union list contains 97 subjects and the Parliament alone has the power to legislate. The State List contains 66 subjects and the States have power to legislate. However, in respect of Concurrent List, which contains 52 subjects, both the Parliament and the State Legislatures have the power to legislate. There are about 200 Central and State Legislation on environmental protection. The most important environmental legislation, passed by the parliament under Art. 249 of the Constitution are The Water (Prevention and Control of Pollution) Act, 1974; The Air (Prevention and Control of Pollution) Act, 1974; The Air (Prevention and Control of Pollution) Act, 1981; and the Environment (Protection) Act, 1986.
3. Fundamental Rights:- Part –III of the Constitution, containing Arts. 12 to 35, deals with fundamental rights. Articles 15(2)(b); Art. 21 and Art. 24 provide for specific provision for environmental protection.
Article 15(2)(b):- According to Art. 15(2)(b), “No citizen shall, on grounds only of religion, race, caste, sex, place of birth or any of them be subjected to any disability, liability, restriction or condition with regard to: the use of wells, bathing ghats, roads and places of public resort, maintained wholly or partly out for state funds or dedicated to the use of general public:. In simple words, Art. 15(2) prohibits discrimination on the ground of sex, race, religion, caste, place of birth etc. to make use of the public places the general public. The public places, which are part and parcel of the human environment should be made available to the public. The preamble to our constitution ensures socialistic pattern of the society and decent standard of life, which can be pollution free environment.
Article 21:- According to Article 21 of the constitution, “no person shall be deprived of his life or personal liberty except according to procedure established by law”.
Article 21 is the heart of the fundamental rights and has received expanded meaning from time to time after the decision of the Supreme Court in Maneka Gandhi vs. Union of India, (AIR 1978 SC 597). Art. 21 guarantees a fundamental right to life –a life of dignity to be lived in a proper environment, free of danger of disease and infection. The right to live in a healthy environment as part of Art. 21 of the Constitution was first recognized in the case of.
Rural Litigation and Entitlement Kendra vs. State of U.P., AIR 1988 SC 2187 (Popularly known as Dehradun Quarrying Case).
It is the first case of this kind in India, involving issues relating to environment and ecological balance. The R.L. & E. Kendra and others in a letter to the Supreme Court complained about the illegal / unauthorized mining in the Missouri, Dehradun belt. As a result, the ecology of the surrounding area was adversely affected and it led to the environmental disorder.
The Supreme Court treated the letter as writ petition under Art. 32 of the Constitution and directed to stop the excavation (illegal mining) under the Environment (Protection) Act, 1986. The respondents contended / argued that the write petition was registered in 1983 and the Environment (Protection) Act was passed in 1986 and hence the criminal proceedings cannot be initiated with retrospective effect. The court rejected the contention of the respondents and held that the provisions of procedural law shall apply to ordinary criminal cases and not to the environmental cases. The court directed the Central and State Governments to take necessary steps to prevent illegal mining and to re-afforesation in the area of mining.
In M.C. Mehta vs. Union of India, AIR 1987 SC 1086 (Popularly known as “Oleum Gas Leak Case”) – The Supreme Court treated the right to live in pollution free environment as a part of fundamental right to life under Art. 21 of the Constitution. Further the A.P. High Court in T. Damodar Rao vs. S.O., Municipal Corporation, Hyderabad, (AIR 1987 A.P. 171) laid down that right to live in healthy environment was specially declared to be part of Art. 21 to the Constitution.
Article 24: Article 24 of the Constitution speaks about exploitation of child labour. It says that “No child below the age of 14 years shall be employed to wok in any factory or mine or engaged in any other hazardous employment” this provisions is certainly in the interest of public health and part of the environment. Further, Art. 39 (e) and 39 (f) under Directive Principles of State Policy provide for the protection of the health and strength of children below the age of 14 years.
In people’s Union for Democratic Rights vs. Union of India, (AIR 1982 SC 1473), the Supreme Court held that the prohibition under Art. 24 could be enforced against any one, be it the State or private individual.
In pursuance of this obligation, parliament enacted the Child Labour (prohibition and Regulation) Act, 1986. The Act prohibits specifically the employment of children in certain industries.
4. Directive Principles of State Policy:- Part IV of the Constitution, Containing Articles 36 to 51, deals with Directive Principles of State Policy. The directive principles form the fundamental feature and are designed to achieve socio economic goals.
Art. 39 (a), which was inserted by the Constitution 42nd Amendment) Act, 1976 provides for Equal Justice and Free Legal Aid. It promotes justice on the basis of equal opportunities. It imposes an imperative duty upon the State to provide free legal aid to the poor litigant so as to secure him equal protection of laws against his well – to – do opponent.
1. Equal right of men and women to adequate means of livelihood.
2. Distribution of ownership and control of the material resources community to the common good.
3. To ensure that the economic system should not result in concentration of wealth and means of production to the common detriment.
4. Equal pay for equal work for both men and women.
5. To protect health and strength of workers and tender age of children and to ensure that they are not forced by economic necessity to entire avocations unsuited to their age or strength; and
6. That children are given opportunities and facilities to develop in a healthy manner and in conditions of freedom and dignity and that childhood and youth are protected against exploitation and against moral and material abandonment.
Article 39(b):- The expression ‘material source’ under Art. 39 (b) means all things, which are capable producing wealth for the community. In includes those, which are already vested in the State but also in the hands of private individuals. Further, the expression ‘distribution’ in Art. 39 (b) does not mean that one’s property is taken over and is distributed to others. It also includes nationalization which is an effective means to prevent concentration of wealth in a few hands so as to benefit the society at large.
Article 39(1):- Art. 39(1) was amended by the Constitution (42nd Amendment) Act, 1976 with a view to emphasize the constructive role of the State with regard to children.
Article 47:- Art. 47 provides that the State shall regard the raising of the level of nutrition and the standard of living of its people and the improvement of public health as among its primary duties. The improvement of public health also includes the protection and improvement of environment without which public health cannot be assured.
Article 48:- It deals with organization of agriculture and animal husbandry. Art. 48 directs the State to take steps to organize agriculture and animal husbandry on modern and scientific lines. In particular, it should take steps for preserving and improving the breeds and prohibiting the slaughter of cows and calves and other milch and draught cattle.
Article 49:-It deals with protection of monuments and places and objects of national importance. Art. 49 requires the State to protect c-very monument or place or object of artistic or historic interest (declared by or under law made by parliament to be of national importance) from spoliation, disfigurement, destruction, removal, disposal or export.
5. Fundamental Duties (51-A):- Art. 51-A was added under the Constitution (42nd Amendment) Act. 1976, which deals with 'Fundamental Duties' under Part IV-A. Art. 51 -A enlists ten fundamental duties designed for restructuring and building a welfare society 'State
Art. 51 -A(g) specifically deals with the fundamental duty with respect to environment. It provides "it shall be the duty of every citizen of India to protect and improve the natural environment including forests, lakes, rivers and wild life and to have compassion for living creatures. (To put it simply Art. 51-A(g) refers to the fundamental duty of every citizen to protect and improve 'natural environment'.
The constitution, being the fundamental law of the land has a binding force on citizens, non – citizens as well as the State. The Fundamental Rights and the Directive Principles of the State Policy underline our national commitment to protect and improve the environment. The courts in India have also given a new interpretation to the constitutional provision relating to protection and improvement of the environment (the intended meaning of the environment in the constitution) may be explained with reference to the following head:
1. The Constitution Forty Second Amendment.
2. Federal System of Govt. (Distribution of Legislative Power).
3. Fundamental Rights.
4. Directive Principles of State Policy; and
5. Fundamental
1. Constitution Forty Second Amendment: - In 1976, under the leadership of the then Prime Minister, Smt. Indira Gandhi, the Constitution (Forty Second Amendment) Act was passed and the provisions relating to the protection of environment for the first time were incorporated by adding a new provision Article 48-A in the Chapter, Directive Principles of State Policy.
According to Article 48-A “the State shall Endeavour to protect and improve the environment and to safeguard the forests and wildlife of the country”.
Further, a new provision Article 51-A in the form of “Fundamental Duties” was also incorporated by the 42nd Amendment. According to the sub-clause (g) of Art. 51-A, “it shall be the duty of every citizen of India to protect and improve the natural environment including forests, lakes, rivers and wildlife and to have compassion for living creatures”. The above two provision impose two-fold responsibilities. On the one hand, it gives directive to the State for protection and improvement of environment, and on the other hand it casts/imposes a duty on every citizen to help in the preservation of natural environment.
2. Federal System of Government (Distribution of Legislative Power):-
From environmental point of view, allocation of legislative authority is very important. The constitution of India deals exhaustively with legislative powers pertaining to environmental law. The legislative powers under the scheme of the constitution is divided into three lists viz., the Union List or List – I, the State List or List –II, the Concurrent List or List – III. Part – XI (Arts. 245-263) of the constitution provides for the distribution of legislative powers between the union and the states. Article 246 distributes the subjects of legislative power in these three lists between the Centre and the States. The union list contains 97 subjects and the Parliament alone has the power to legislate. The State List contains 66 subjects and the States have power to legislate. However, in respect of Concurrent List, which contains 52 subjects, both the Parliament and the State Legislatures have the power to legislate. There are about 200 Central and State Legislation on environmental protection. The most important environmental legislation, passed by the parliament under Art. 249 of the Constitution are The Water (Prevention and Control of Pollution) Act, 1974; The Air (Prevention and Control of Pollution) Act, 1974; The Air (Prevention and Control of Pollution) Act, 1981; and the Environment (Protection) Act, 1986.
3. Fundamental Rights:- Part –III of the Constitution, containing Arts. 12 to 35, deals with fundamental rights. Articles 15(2)(b); Art. 21 and Art. 24 provide for specific provision for environmental protection.
Article 15(2)(b):- According to Art. 15(2)(b), “No citizen shall, on grounds only of religion, race, caste, sex, place of birth or any of them be subjected to any disability, liability, restriction or condition with regard to: the use of wells, bathing ghats, roads and places of public resort, maintained wholly or partly out for state funds or dedicated to the use of general public:. In simple words, Art. 15(2) prohibits discrimination on the ground of sex, race, religion, caste, place of birth etc. to make use of the public places the general public. The public places, which are part and parcel of the human environment should be made available to the public. The preamble to our constitution ensures socialistic pattern of the society and decent standard of life, which can be pollution free environment.
Article 21:- According to Article 21 of the constitution, “no person shall be deprived of his life or personal liberty except according to procedure established by law”.
Article 21 is the heart of the fundamental rights and has received expanded meaning from time to time after the decision of the Supreme Court in Maneka Gandhi vs. Union of India, (AIR 1978 SC 597). Art. 21 guarantees a fundamental right to life –a life of dignity to be lived in a proper environment, free of danger of disease and infection. The right to live in a healthy environment as part of Art. 21 of the Constitution was first recognized in the case of.
Rural Litigation and Entitlement Kendra vs. State of U.P., AIR 1988 SC 2187 (Popularly known as Dehradun Quarrying Case).
It is the first case of this kind in India, involving issues relating to environment and ecological balance. The R.L. & E. Kendra and others in a letter to the Supreme Court complained about the illegal / unauthorized mining in the Missouri, Dehradun belt. As a result, the ecology of the surrounding area was adversely affected and it led to the environmental disorder.
The Supreme Court treated the letter as writ petition under Art. 32 of the Constitution and directed to stop the excavation (illegal mining) under the Environment (Protection) Act, 1986. The respondents contended / argued that the write petition was registered in 1983 and the Environment (Protection) Act was passed in 1986 and hence the criminal proceedings cannot be initiated with retrospective effect. The court rejected the contention of the respondents and held that the provisions of procedural law shall apply to ordinary criminal cases and not to the environmental cases. The court directed the Central and State Governments to take necessary steps to prevent illegal mining and to re-afforesation in the area of mining.
In M.C. Mehta vs. Union of India, AIR 1987 SC 1086 (Popularly known as “Oleum Gas Leak Case”) – The Supreme Court treated the right to live in pollution free environment as a part of fundamental right to life under Art. 21 of the Constitution. Further the A.P. High Court in T. Damodar Rao vs. S.O., Municipal Corporation, Hyderabad, (AIR 1987 A.P. 171) laid down that right to live in healthy environment was specially declared to be part of Art. 21 to the Constitution.
Article 24: Article 24 of the Constitution speaks about exploitation of child labour. It says that “No child below the age of 14 years shall be employed to wok in any factory or mine or engaged in any other hazardous employment” this provisions is certainly in the interest of public health and part of the environment. Further, Art. 39 (e) and 39 (f) under Directive Principles of State Policy provide for the protection of the health and strength of children below the age of 14 years.
In people’s Union for Democratic Rights vs. Union of India, (AIR 1982 SC 1473), the Supreme Court held that the prohibition under Art. 24 could be enforced against any one, be it the State or private individual.
In pursuance of this obligation, parliament enacted the Child Labour (prohibition and Regulation) Act, 1986. The Act prohibits specifically the employment of children in certain industries.
4. Directive Principles of State Policy:- Part IV of the Constitution, Containing Articles 36 to 51, deals with Directive Principles of State Policy. The directive principles form the fundamental feature and are designed to achieve socio economic goals.
Art. 39 (a), which was inserted by the Constitution 42nd Amendment) Act, 1976 provides for Equal Justice and Free Legal Aid. It promotes justice on the basis of equal opportunities. It imposes an imperative duty upon the State to provide free legal aid to the poor litigant so as to secure him equal protection of laws against his well – to – do opponent.
1. Equal right of men and women to adequate means of livelihood.
2. Distribution of ownership and control of the material resources community to the common good.
3. To ensure that the economic system should not result in concentration of wealth and means of production to the common detriment.
4. Equal pay for equal work for both men and women.
5. To protect health and strength of workers and tender age of children and to ensure that they are not forced by economic necessity to entire avocations unsuited to their age or strength; and
6. That children are given opportunities and facilities to develop in a healthy manner and in conditions of freedom and dignity and that childhood and youth are protected against exploitation and against moral and material abandonment.
Article 39(b):- The expression ‘material source’ under Art. 39 (b) means all things, which are capable producing wealth for the community. In includes those, which are already vested in the State but also in the hands of private individuals. Further, the expression ‘distribution’ in Art. 39 (b) does not mean that one’s property is taken over and is distributed to others. It also includes nationalization which is an effective means to prevent concentration of wealth in a few hands so as to benefit the society at large.
Article 39(1):- Art. 39(1) was amended by the Constitution (42nd Amendment) Act, 1976 with a view to emphasize the constructive role of the State with regard to children.
Article 47:- Art. 47 provides that the State shall regard the raising of the level of nutrition and the standard of living of its people and the improvement of public health as among its primary duties. The improvement of public health also includes the protection and improvement of environment without which public health cannot be assured.
Article 48:- It deals with organization of agriculture and animal husbandry. Art. 48 directs the State to take steps to organize agriculture and animal husbandry on modern and scientific lines. In particular, it should take steps for preserving and improving the breeds and prohibiting the slaughter of cows and calves and other milch and draught cattle.
Article 49:-It deals with protection of monuments and places and objects of national importance. Art. 49 requires the State to protect c-very monument or place or object of artistic or historic interest (declared by or under law made by parliament to be of national importance) from spoliation, disfigurement, destruction, removal, disposal or export.
5. Fundamental Duties (51-A):- Art. 51-A was added under the Constitution (42nd Amendment) Act. 1976, which deals with 'Fundamental Duties' under Part IV-A. Art. 51 -A enlists ten fundamental duties designed for restructuring and building a welfare society 'State
Art. 51 -A(g) specifically deals with the fundamental duty with respect to environment. It provides "it shall be the duty of every citizen of India to protect and improve the natural environment including forests, lakes, rivers and wild life and to have compassion for living creatures. (To put it simply Art. 51-A(g) refers to the fundamental duty of every citizen to protect and improve 'natural environment'.



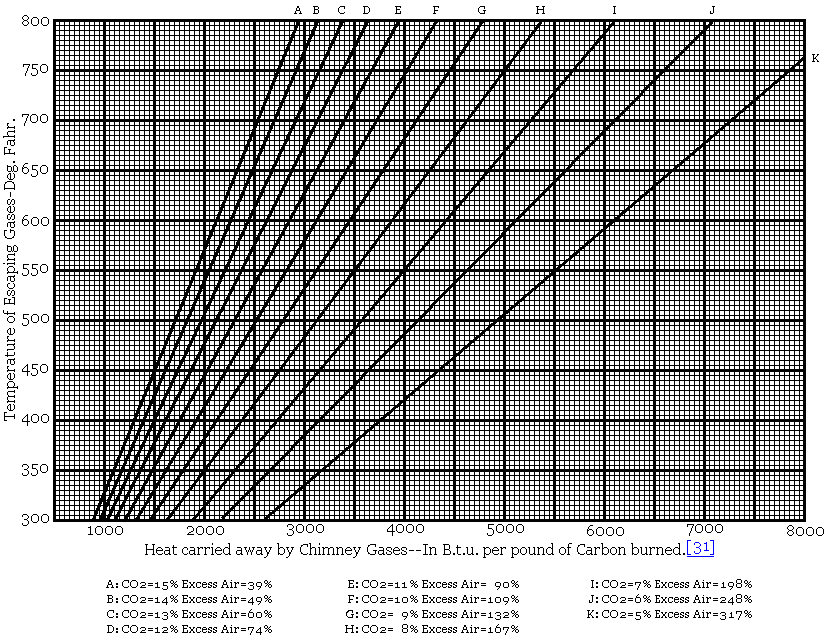
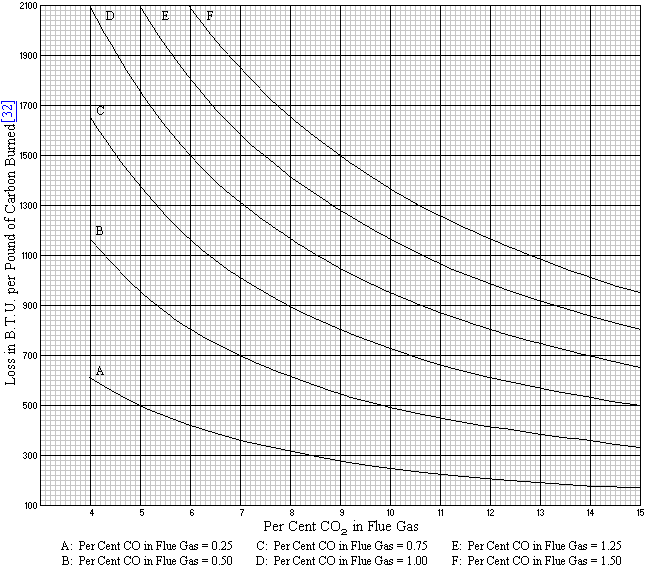



 The outdoor pollution alone kills 1.09 lakh adults and 7,513 children every year in India, while about six per cent (nearly Rs 3.75 lakh crore) of the country’s gross domestic product (GDP) is getting lost due to a ruining environment. The numbers were flagged in the recently released World Bank study that was commissioned by the central government.
The outdoor pollution alone kills 1.09 lakh adults and 7,513 children every year in India, while about six per cent (nearly Rs 3.75 lakh crore) of the country’s gross domestic product (GDP) is getting lost due to a ruining environment. The numbers were flagged in the recently released World Bank study that was commissioned by the central government.

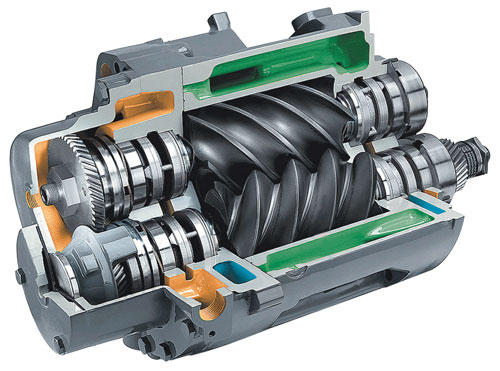 It’s not just the cost of lost production either. A compressor failure in a single part of the refinery can cost tens of thousands of dollars a day in lost revenue, with similar amounts to rebuild a compressor, and hundreds of thousands of dollars for a replacement. There’s also the cost of maintaining spares.
It’s not just the cost of lost production either. A compressor failure in a single part of the refinery can cost tens of thousands of dollars a day in lost revenue, with similar amounts to rebuild a compressor, and hundreds of thousands of dollars for a replacement. There’s also the cost of maintaining spares.
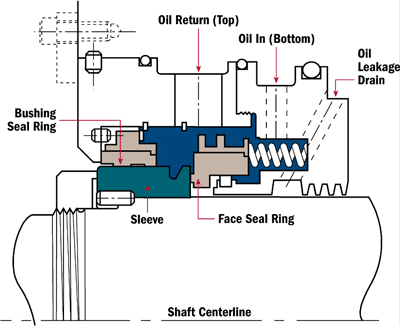
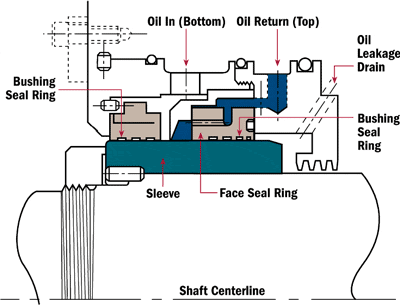
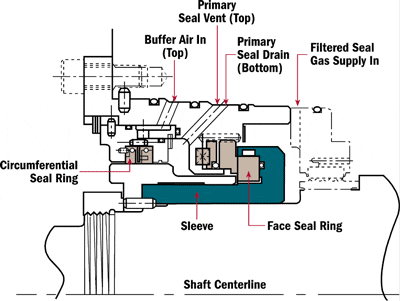

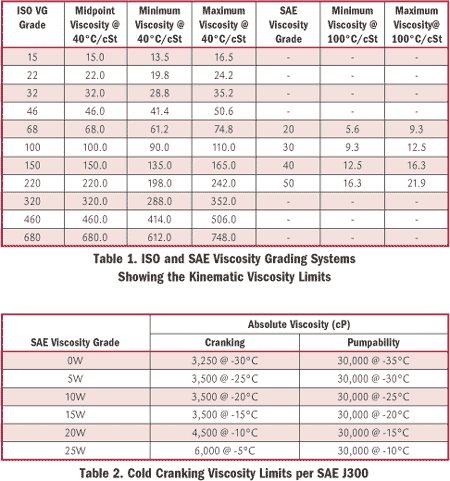

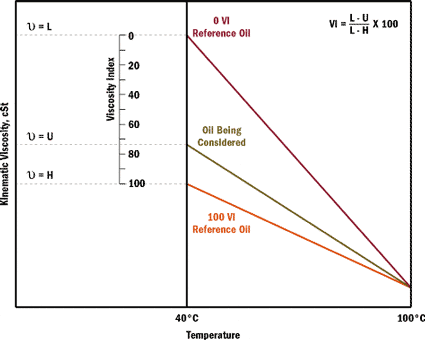
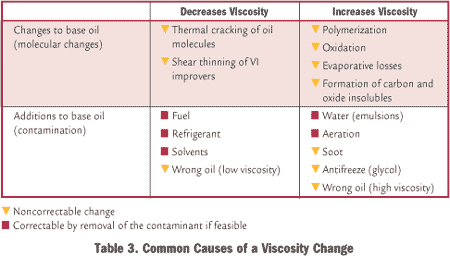
 “We are using an onsite viscometer to measure viscosity at 40 degrees C. At the same time, our lube supplier is testing samples from the same system regularly. However, our viscosity numbers are often up to 10 percent different from the lube suppliers. What are we doing wrong?”
“We are using an onsite viscometer to measure viscosity at 40 degrees C. At the same time, our lube supplier is testing samples from the same system regularly. However, our viscosity numbers are often up to 10 percent different from the lube suppliers. What are we doing wrong?”






